Report a Patient Safety Concern or File a Complaint
9 hours ago If you have a medical emergency, please call 911. If you are having thoughts of harming yourself, please call the National Suicide Prevention Lifeline at 1-800-273-TALK (8255). Online: Submit a NEW patient safety event or concern. >> Go To The Portal
Reporting Patient Safety Events
- Background. Patient safety event reporting systems are ubiquitous in hospitals and are a mainstay of efforts to detect patient safety events and quality problems.
- Characteristics of Incident Reporting Systems. ...
- Limitations of Event Reporting. ...
- Using Event Reports to Improve Safety. ...
- Current Context. ...
Full Answer
How do you write a patient incident report?
In order to record the most accurate account of the incident, maintain an objective tone. Do not include assumptions or assign blame; just write down the facts. Where possible, include direct quotes from the patient and/or other involved parties. The higher your quality of writing, the more valuable your patient incident report will be.
Why is it important to report patient safety events?
The reporting of all patient safety events, even those that don’t reach the patient, allows the DoD PSP to identify, analyze and learn from the sequence of events that may potentially lead to errors before they affect patients.
How do I file a complaint under the Patient Safety Act?
Your complaint must: Name the person that is the subject of the complaint and describe the act or acts believed to be in violation of the Patient Safety Act requirement to keep PSWP confidential
What is a patient safety information report (pswp)?
PSWP is any information: PSWP may identify patients, health care providers and individuals that report medical errors or other patient safety events. This PSWP is confidential and may only be disclosed in certain very limited situations.
How do you report a patient event or a safety hazard in the environment?
Dial the Hotline (310) 825-9797 Follow the instructions by the voice operator and choose from the menu. A manager on call will respond based on the type of incident.
What organization is responsible for patient safety?
A Patient Safety Organization (PSO) works with healthcare providers to help them improve patient safety and healthcare quality and encourage a culture of safety.
What is patient safety report?
Patient Safety Reporting (PSR) gives military treatment facility personnel the ability to anonymously report medical events that impact the safety of patients.
How do you address a patient safety?
5 Patient-Centered Strategies to Improve Patient SafetyAllow patients access to EHR data, clinician notes. ... Care for hospital environment. ... Create a safe patient experience. ... Create simple and timely appointment scheduling. ... Encourage family and caregiver engagement.
What reports are encouraged as a result of the Patient Safety and Quality Improvement Act?
What reports are encourage as a result of the patient safety and quality improvement act? near misses, unsafe conditions, adverse events, events the threaten patient safety.
Which governmental Agency monitors safety practices in the healthcare environment?
The FDA is responsible for protecting the public health by assuring the safety, efficacy, and security of human and veterinary drugs, biological products, medical devices, our nation's food supply, cosmetics, and products that emit radiation.
What is the name of the system where incident reporting has to be done?
Incident Reporting Systems (IRS) are a cornerstone for improving patient safety.
Why reporting is necessary for patient safety?
Reporting systems (frequently referred to as reporting and learning systems) capture patient safety concerns, hazards and/or incidents and are meant to trigger action, facilitate communication, response, learning and improvement.
What is patient safety in healthcare?
What is Patient Safety? Patient Safety is a health care discipline that emerged with the evolving complexity in health care systems and the resulting rise of patient harm in health care facilities. It aims to prevent and reduce risks, errors and harm that occur to patients during provision of health care.
What is the nurses role in patient safety?
From a patient safety perspective, a nurse's role includes monitoring patients for clinical deterioration, detecting errors and near misses, understanding care processes and weaknesses inherent in some systems, identifying and communicating changes in patient condition, and performing countless other tasks to ensure ...
What are some patient safety issues?
The 10 patient safety concerns every health care worker needs to know aboutMedication errors. ... Diagnostic errors. ... Patient discharge errors. ... Workplace safety issues. ... Aging hospital facility issues. ... Reprocessing issues. ... Sepsis. ... "Super" superbugs.More items...
What are 5 safety concerns in healthcare?
Patient safety issues and concernsMedication/drug errors. ... Healthcare-associated infections. ... Surgical errors and postoperative complications. ... Diagnostic errors. ... Laboratory/blood testing errors. ... Fall injuries. ... Communication errors. ... Patient identification errors.
About Patient Safety Confidentiality
OCR enforces the confidentiality provisions of the Patient Safety and Quality Improvement Act of 2005 (Patient Safety Act) and the Patient Safety and Quality Improvement Rule (Patient Safety Rule).
What is PSWP?
Assembled or developed by a health care provider for reporting to a Patient Safety Organization (PSO) that is listed by the HHS Agency for Healthcare Research and Quality (AHRQ) and is documented as being within the provider’s patient safety evaluation system for reporting to a PSO
Complaint Requirements
Anyone can file a patient safety confidentiality complaint. If you believe that a person or organization shared PSWP, you may file a complaint with OCR. Your complaint must:
File a Patient Safety Confidentiality Complaint
File a Complaint Using the Patient Safety Confidentiality Complaint Form Package
How OCR Investigates Your Complaint
OCR will investigate complaints that allege potential violations of the Rule. To the extent practicable, OCR will provide technical assistance and seek informal resolution of complaints involving the inappropriate sharing of PSWP through voluntary compliance from the responsible person, entity, or organization.
What is patient safety event reporting?
Patient safety event reporting systems are ubiquito us in hospitals and are a mainstay of efforts to detect patient safety events and quality problems. Incident reporting is frequently used as a general term for all voluntary patient safety event reporting systems, which rely on those involved in events to provide detailed information. Initial reports often come from the frontline personnel directly involved in an event or the actions leading up to it (e.g., the nurse, pharmacist, or physician caring for a patient when a medication error occurred), rather than management or patient safety professionals. Voluntary event reporting is therefore a passive form of surveillance for near misses or unsafe conditions, in contrast to more active methods of surveillance such as direct observation of providers or chart review using trigger tools. The Patient Safety Primer Detection of Safety Hazards provides a detailed discussion of other methods of identifying errors and latent safety problems.
How is event reporting used in health care?
A 2016 article contrasted event reporting in health care with event reporting in other high-risk industries (such as aviation), pointing out that event reporting systems in health care have placed too much emphasis on collecting reports instead of learning from the events that have been reported. Event reporting systems are best used as a way of identifying issues that require further, more detailed investigation. While event reporting utilization can be a marker of a positive safety culture within an organization, organizations should resist the temptation to encourage event reporting without a concrete plan for following up on reported events. A PSNet perspective described a framework for incorporating voluntary event reports into a cohesive plan for improving safety. The framework emphasizes analysis of the events and documenting process improvements arising from event analysis, rather than encouraging event reporting for its own sake.
What is the Patient Safety and Quality Improvement Act?
The legislation provides confidentiality and privilege protections for patient safety information when health care providers work with new expert entities known as Patient Safety Organizations (PSOs). Health care providers may choose to work with a PSO and specify the scope and volume of patient safety information to share with a PSO. Because health care providers can set limits on the ability of PSOs to use and share their information, this system does not follow the pattern of traditional voluntary reporting systems. However, health care providers and PSOs may aggregate patient safety event information on a voluntary basis, and AHRQ will establish a network of patient safety databases that can receive and aggregate nonidentifiable data that are submitted voluntarily. AHRQ has also developed Common Formats —standardized definitions and reporting formats for patient safety events—in order to facilitate aggregation of patient safety information. Since their initial release in 2009, the Common Formats have been updated and expanded to cover a broad range of safety events.
Why are event reports limited?
The spectrum of reported events is limited, in part due to the fact that physicians generally do not utilize voluntary event reporting systems.
Is there a voluntary event reporting system?
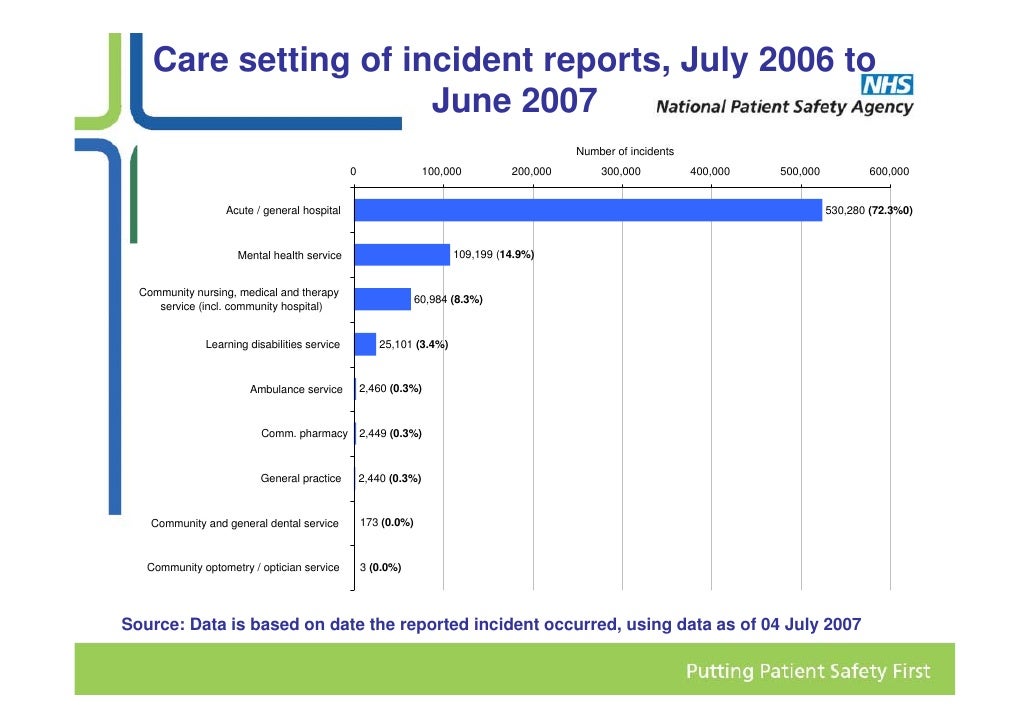
Voluntary event reporting systems need not be confined to a single hospital or organization. The United Kingdom's National Patient Safety Agency maintains the National Reporting and Learning System, a nationwide voluntary event reporting system, and the MEDMARX voluntary medication error reporting system in the U.S.
How long after incident should you report a patient?
Patient incident reports should be completed no more than 24 to 48 hours after the incident occurred. You may even want to file the report by the end of your shift to ensure you remember all the incident’s important details. RELATED: Near Miss Reporting: Why It’s Important.
What to include in an incident report?
Every facility has different needs, but your incident report form could include: 1 Date, time and location of the incident 2 Name and address of the facility where the incident occurred 3 Names of the patient and any other affected individuals 4 Names and roles of witnesses 5 Incident type and details, written in a chronological format 6 Details and total cost of injury and/or damage 7 Name of doctor who was notified 8 Suggestions for corrective action
Why is it important to review patient incidents?
Reviewing incidents helps administrators know what risk factors need to be corrected within their facilities , reducing the chance of similar incidents in the future.
Why is it important to know that an incident has occurred?
Knowing that an incident has occurred can push administrators to correct factors that contributed to the incident. This reduces the risk of similar incidents in the future. Quality control. Medical facilities want to provide the best care and customer service possible.
Why do we use resolved patient incident reports?
Using resolved patient incident reports to train new staff helps prepare them for real situations that could occur in the facility. Similarly, current staff can review old reports to learn from their own or others’ mistakes and keep more incidents from occurring. Legal evidence.
How long does it take to file a patient incident report?
Patient incident reports should be completed no more than 24 to 48 hours after the incident occurred.
Why is it important to document an incident?
Even if an incident seems minor or didn’t result in any harm, it is still important to document it. Whether a patient has an allergic reaction to a medication or a visitor trips over an electrical cord, these incidents provide insight into how your facility can provide a better, safer environment.
Where to file a patient safety event?
You can file this report by going to www.jointcommission.org, and using the “Report a Patient Safety Event” link in the “Action Center” of the homepage. You can also file by fax to 630-792-5636.
Does CVS have a notice of patient rights?
Every CVS MinuteClinic should provide you with a Notice of Patient Rights or at least have one posted and available to you. This notice states that you have the right to be informed of the procedure for submitting a complaint about MinuteClinic and/or the quality of care you have received.
Complaints about the quality of your care
Contact your Beneficiary and Family Centered Care Quality Improvement Organization (BFCC-QIO) for complaints about the quality of care you got from a Medicare provider.
note
For questions about a specific service you got, look at your Medicare Summary Notice (MSN) or log into your secure Medicare account . You can file an appeal if you disagree with a coverage or payment decision made by one of these:

Background
Characteristics of Incident Reporting Systems
- An effective event reporting system should have four key attributes: While traditional event reporting systems have been paper based, technological enhancements have allowed the development of Web-based systems and systems that can receive information from electronic medical records. Specialized systems have also been developed for specific settings, such as th…
Limitations of Event Reporting
- The limitations of voluntary event reporting systems have been well documented. Event reports are subject to selection bias due to their voluntary nature. Compared with medical record review and direct observation, event reports capture only a fraction of events and may not reliably identify serious events. The spectrum of reported events is limited, in part due to the fact that ph…
Using Event Reports to Improve Safety
- A 2016 article contrasted event reporting in health care with event reporting in other high-risk industries (such as aviation), pointing out that event reporting systems in health care have placed too much emphasis on collecting reports instead of learning from the events that have been reported. Event reporting systems are best used as a way of identifying issues that require furth…
Current Context
- At the national level, regulations implementing the Patient Safety and Quality Improvement Act became effective on January 19, 2009. The legislation provides confidentiality and privilege protections for patient safety information when health care providers work with new expert entities known as Patient Safety Organizations (PSOs). Health care providers may choose to wo…
Popular Posts:
- 1. shore diagnosics easton md patient portal
- 2. dr romito norwich ct patient portal
- 3. eastside family medicine patient portal frankfort ky
- 4. wilmington heath patient portal
- 5. arizona eye consultants on speedway patient portal
- 6. iha patient portal hamburg
- 7. digestive disease associates patient portal
- 8. hippa patient privacy incident report georgia
- 9. dermlasercenter.ema.md patient portal
- 10. military genesis patient portal