, neuroderm-announces-completion-patient ... - - Chemdiv
31 hours ago , neuroderm-announces-completion-patient-enrollment-treatment-phase-ii-trial-nd0612h-advanced-parkinsons-disease - Chemdiv >> Go To The Portal
Why Neuroderm for Parkinson's disease?
, neuroderm-announces-completion-patient-enrollment-treatment-phase-ii-trial-nd0612h-advanced-parkinsons-disease - Chemdiv
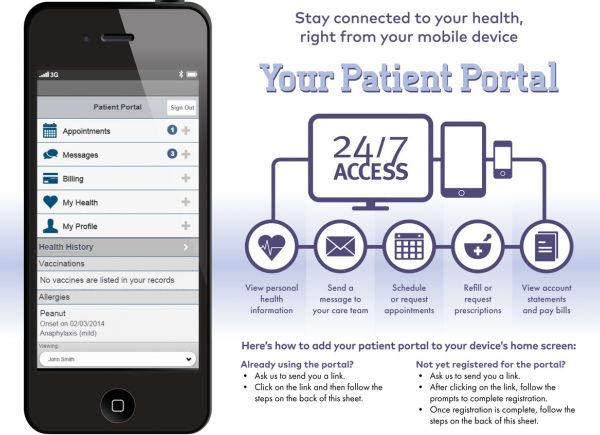
How do I log in to my Patient Portal?
NeuroDerm has developed two doses of ND0612: the low-dose form is used to treat moderate stage Parkinson’s patients who can no longer effectively control their motor complications with oral levodopa, and a higher dose for the treatment of severe Parkinson’s patients for whom oral drugs are no longer effective.ND0612H was designed to be a safer, more effective and …
How is neurodermatitis diagnosed and treated?
Sep 19, 2016 · NeuroDerm has started enrolling Parkinson’s disease (PD) patients in iNDiGO , a Phase 3 clinical trial evaluating the effectiveness of ND0612L, the company’s levodopa/carbidopa (LD/CD) subcutaneous liquid formulation. Levodopa and dopamine agonists, the first line of defense against Parkinson’s disease, are drugs commonly used to improve ...
What should I do if I have technical issues with patient portal?
Mar 06, 2017 · NeuroDerm’s Phase 2 Parkinson’s disease (PD) trial of ND0612H — a continuous infusion of levodopa and carbidopa delivered by a small belt pump — has met both primary and key secondary endpoints by demonstrating that the treatment effectively reduced “off-time” and increased the number of patients with advanced Parkinson’s who experienced treatment …

What are the side effects of Parkinson's?
Serious side effects include hallucinations and delusions, unexpectedly falling asleep, unusual urges, high fever, and confusion related to medication withdrawal. Note: Parkinson’s News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment.
Does levodopa help with dyskinesia?
Once in the blood, levodopa travels to ...
What is ND0612L?
NeuroDerm has developed two doses of ND0612: the low-dose ( ND0612L) form is used to treat moderate stage Parkinson’s patients who can no longer effectively control their motor complications with oral levodopa, and a higher dose ( ND0612H) for the treatment of severe Parkinson’s patients for whom oral drugs are no longer effective.
Does levodopa cause headaches?
Other common adverse events included local bleeding at the infusion sites and headache. Levodopa and carbidopa are known to be associated with adverse events, the most common being dyskinesia, nausea, vomiting, dizziness, low blood pressure upon rising, headache, sleeplessness, dry mouth, anxiety, and constipation.
What is the best medicine for itching?
Because anxiety and stress can trigger neurodermatitis, anti-anxiety drugs may help prevent the itchiness. Medicated patches. For stubborn itching, your doctor may suggest topical lidocaine 5 percent or capsaicin 8 percent patches.
How to diagnose neurodermatitis?
Diagnosis. Your doctor may diagnose neurodermatitis by examining the affected skin and determining whether you've been itching and scratching. To rule out other causes, he or she may take a small sample of the affected skin (skin biopsy) for testing.
What to do if your itching persists despite treatment?
If your itching persists despite treatment, your doctor may suggest a nontraditional approach. For example, in small studies some people whose symptoms didn't improve with corticosteroid use did report success with the following treatments. OnabotulinumtoxinA (Botox) injection.
How to stop itching from a scab?
The itching may be intense, but avoiding rubbing and scratching is key to controlling your condition and preventing a recurrence. Apply cool, wet compresses. These may soothe the skin and relieve the itch.
How to stop itching from a swollen ear?
Try over-the-counter medications. Apply an anti-itch cream or lotion to the affected area. A hydrocortisone cream can temporarily relieve the itch. An oral antihistamine, such as diphenhydramine can relieve severe itching and help you sleep.
How to stop scratching while sleeping?
Bandages or dressings can help protect the skin and prevent scratching. These may be especially useful if you scratch during your sleep. Keep your nails trimmed. Short nails may do less damage to the skin, especially if you tend to scratch while you're asleep. Take short, warm baths and moisturize your skin.
Diagnosis
- Your doctor may diagnose neurodermatitis by examining the affected skin and determining whether you've been itching and scratching. To rule out other causes, he or she may take a small sample of the affected skin (skin biopsy) for testing.
Treatment
- Treatment is aimed at controlling the itching, preventing scratching and addressing underlying causes. 1. Anti-itch medicated creams.If over-the-counter corticosteroid cream isn't helping, your doctor may prescribe a stronger corticosteroid or a nonsteroidal anti-itch product. A calcineurin inhibitor (tacrolimus) ointment may help if the vulva is involved. 2. Corticosteroid injections.You…
Lifestyle and Home Remedies
- These self-care measures can help you manage neurodermatitis: 1. Stop rubbing and scratching.The itching may be intense, but avoiding rubbing and scratching is key to controlling your condition and preventing a recurrence. 2. Apply cool, wet compresses.These may soothe the skin and relieve the itch. Putting a cool, wet compress on the affected skin...
Preparing For Your Appointment
- You may start by seeing your primary care physician. He or she may refer you to a doctor who specializes in skin conditions (dermatologist). Here's some information to help you get ready for your appointment.
Popular Posts:
- 1. patient portal joshi
- 2. st. luke's hospital east lee's suimmit missouri patient portal
- 3. dr. vijay singh mt. laurel nj patient portal
- 4. comprehsive pain specialists saginaw mi patient portal
- 5. murray calloway patient portal
- 6. woburn pediatrics patient portal
- 7. st joe's hospital syracuse patient portal
- 8. christus saint michael s patient portal
- 9. midlands internal medicine patient portal
- 10. dmc org patient portal