Writing A Case Report - Michigan State University
2 hours ago If you need to select on your own, here are some strategies: 1. Do a PubMed search. https://pubmed.ncbi.nlm.nih.gov/. a. Do a search for a topic, disease or other feature of your case report. b. When the results appear, on the left side of the page is a limiter for "article type". >> Go To The Portal
What is the purpose of a patient case report?
Patient case reports are valuable resources of new and unusual information that may lead to vital research. Patient case reports are valuable resources of new and unusual information that may lead to vital research. How to write a patient case report
How do you confirm that a case report is valid?
The author must confirm that the case report is valid by ensuring the accuracy of the data presented and by establishing a temporal and causal relationship. For drug-induced adverse effects, a validated nomogram to establish the probability of causality such as the Naranjo nomogram must be used.8
How do you write a patient perspective in a case report?
The patient should share their perspective on the treatment (s) they received in one to two paragraphs. It is often best to ask for informed consent and the patient’s perspective before you begin writing your case report.
What should be included in a medical case report?
It describes in details the patient’s history, signs, symptoms, test results, diagnosis, prognosis and treatment. It also contains a short literature review, discusses the importance of the case and how it improves the existing knowledge on the subject.
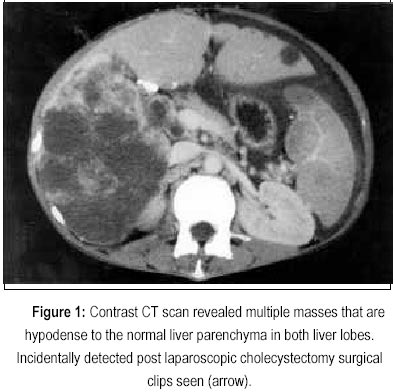
What should be included in the abstract of a patient case report?
The abstract of a patient case report should succinctly include the four sections of the main text of the report. The introduction section should provide the subject, purpose, and merit of the case report.
What is patient case report?
Patient case reports are valuable resources of new and unusual information that may lead to vital research.
What happened to Gage after the accident?
After the accident, Gage became profane, rough and disrespectful to the extent that he was no longer tolerable to people around him . So he lost his job and his family.
Why are case reports important?
Case reports are great to get first impressions that can generate new hypotheses (e.g. detecting a potential side effect of a drug) or challenge existing ones (e.g. shedding the light on the possibility of a different biological mechanism of a disease).
How does a case report affect us?
In his book Against Empathy: The Case for Rational Compassion, Paul Bloom explains how a powerful story affects our emotions, can distort our judgement and even lead us to make bad moral choices.
Why is a control group important?
A control group is important to eliminate the role of external factors which can interfere with the relationship between exposure and disease. Unmeasured Confounding caused by variables that influence both the exposure and the disease. A case report can have a powerful emotional effect (see examples of case reports below).
What is an example of recovery from the passage of an iron bar through the head?
This is an interesting case of a construction foreman named Phineas Gage. [ Source] In 1848, due to an explosion at work, an iron bar passed through his head destroying a large portion of his brain’s frontal lobe.
What is a case report?
Case Report: A Beginner’s Guide with Examples. A case report is a descriptive study that documents an unusual clinical phenomenon in a single patient. It describes in details the patient’s history, signs, symptoms, test results, diagnosis, prognosis and treatment.
Why do we need case reports?
Case reports offer, in general a fast, easy and cheap way to report an unusual observation or a rare event in a clinical setting, as these have very small probability of being detected in an experimental study because of limitations on the number of patients that can be included.
How many paragraphs should a patient share their perspective?
The patient should share their perspective on the treatment (s) they received in one to two paragraphs. It is often best to ask for informed consent and the patient’s perspective before you begin writing your case report. Appendices (If indicated). Submission to a scientific journal.
What should be included in a short acknowledgement?
Acknowledgements. A short acknowledgements section should mention funding support or conflicts of interest, if applicable.
What is follow up and outcomes?
Follow-up and outcomes: describe the clinical course of the episode of care during follow-up visits including (1) intervention modification, interruption, or discontinuation; (2) intervention adherence and how this was assessed; and (3) adverse effects or unanticipated events. Regular patient report outcome measurement surveys such as PROMIS® may be helpful.
How many paragraphs should a conclusion be?
The conclusion, usually one paragraph, offers the most important findings from the case without references.
Can you submit a case report to a scientific journal?
Follow author guidelines and journal submission requirements when writing and submitting your case report to a scientific journal. You may wish to contact the journal before submitting your manuscript. (Download a partial list of journals that accept case reports.) Journals that do not explicitly accept case reports may publish case reports as components of other articles. Online training in writing case reports is available from Scientific Writing in Health and Medicine (SWIHM).
Is it easier to write sections of a case report in a different sequence than the order in which the sections?
Know that it is easier to write the sections of a case report in a different sequence than the order in which the sections appear in a published case report.
Is it easy to write a case report?
Writing a case report accurately and transparently is not easy. We provide online training in writing case reports at Scientific Writing in Health and Medicine (SWIHM) which includes access to CARE-writer, an online app that can be used to write case reports or case report preprints.
What is PHI in healthcare?
This is known as safe harbor de-identification.
What is a case report?
A case report is an unsystematic clinical observation that states the outcome or response of a single patient to a diagnostic strategy or treatment . Case reports serve to document and share novel cases amongst the medical community for educational purposes.
When safe harbor de-identification is not possible or the opportunity to identify the patient exists, even after de-ident?
When safe harbor de-identification is not possible or the opportunity to identify the patient exists, even after de-identification, the expert determination method for de-identification can be considered. For purposes of de-identification, an expert is defined as: A person with appropriate knowledge of and experience with generally accepted statistical and scientific principles and methods for rendering information not individually identifiable:
When case reports describe or discuss unique or rare circumstances, as they often do, it may be difficult or impossible to answer?
When case reports describe or discuss unique or rare circumstances, as they often do, it may be difficult or impossible to de-identify those cases such that there is no reasonable expectation that the individuals included can be identified, so patient authorization generally would be required.
Is PHI de-identified under HIPAA?
It is important to understand that determining whether data are de-identified under HIPAA is a more restrictive determination than determining whether private information is individually identifiable under the Common Rule. The HIPAA rule considers PHI as any information that may identify an individual; was created or received by a member of a HIPAA covered entity; and relates to the individual's past, present, or future physical/mental health or condition, health care, or payment for health care. HIPAA recognizes two methods for de-identification of data.
What is the difference between a paper CRF and an improvised electronic CRF?
There are two types of CRFs used in clinical research, that is, traditional paper CRF and improvised electronic CRF (eCRF). Paper CRF is the traditional way of data capture and a better option if studies are small or vary in design, whereas eCRFs are considered if studies are large with similar designs.[5]
Why are eCRFs preferred over paper CRFs?
In the current global scenario, eCRFs are preferred over paper CRFs as they are less time-consuming, and also encourage the sponsor/pharmaceutical company to carry out large multicentric studies at the same time due to the ease of administration. It is designed in such a way that data entry can be done with zero/minimal errors. Moreover, the regulatory authorities are readily accepting submissions in which validated electronic data capture (EDC) systems are used.[6]
What is the primary objective of CRF design?
Primary objective of CRF designing is to gather complete and accurate data by avoiding duplication and facilitating transcription of data from source documents onto the CRF.
Why should CRF layout be uncrowded?
In order to enhance easy reading/understanding and accurate data entry, an uncrowded CRF layout should be preferred. Placing too many details on the same page, makes the CRF look cluttered and makes data entry difficult, which eventually leads to increase in data discrepancies.
What is CRF design?
CRF design should be standardized to address the needs of all users such as investigator, site coordinator, study monitor, data entry personnel, medical coder and statistician. Data should be organized in a format that facilitates and simplifies data analysis.
What is a CRF in clinical research?
Case report form (CRF) is a specialized document in clinical research. It should be study protocol driven, robust in content and have material to collect the study specific data. Though paper CRFs are still used largely, use of electronic CRFs (eCRFS) are gaining popularity due to the advantages they offer such as improved data quality, ...
Why should case report form be standardized?
Case report form design should be standardized to address the needs of all those who handle the data such as investigator, data manager, biostatistician , clinical research monitor/coordinator, database developer/programmer and data entry personnel etc. An effective CRF design would always be user friendly. Moreover, it should capture legible, consistent and valid data, thereby, reducing query generations.[7] While designing the CRFs, design standards should be adhered to for improving the quality of data collected. Hence, data should be organized in a format that facilitates data analysis and makes it simplified.
What Is a Patient Care Report?
We often hear of care reports based on by medical teams or by medical authorities. Yet, we are not sure how this differs from the kind of report that is given to us by the same people. So this is the time to make it as clear as possible.
How to Write a Patient Care Report?
Where do you even begin when you write a patient care report? A lot of EMS or EMTs do know how to write one since they are trained to do so.
What is a patient care report?
A patient care report is a document made mostly by the EMS or EMTs. This documented report is done after getting the call. This consists of the information necessary for the assessment and evaluation of a patient’s care.
What should not be written in a patient care report?
What should be avoided in a patient care report is making up the information that is not true to the patient. This is why you have to be very careful and very meticulous when writing these kinds of reports. Every detail counts.
Who is in charge of reading the patient care report?
The person or the people who will be reading the report are mostly medical authorities. When you are going to be passing this kind of report, make sure that you have all the information correctly. One wrong information can cause a lot of issues and problems.
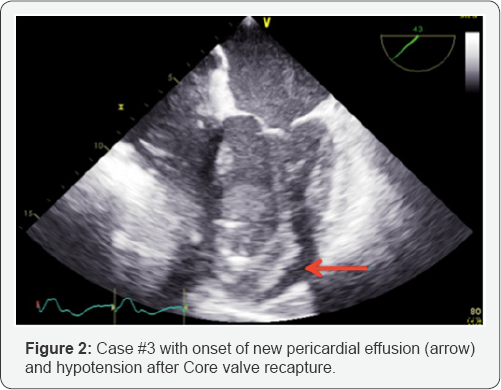
Advantages of Case Reports
- Case reports offer, in general a fast, easy and cheap way to report an unusual observation or a rare event in a clinical setting, as these have very small probability of being detected in an experimental study because of limitations on the number of patients that can be included. These events deserve to be reported since they might provide insights on some exceptions to general rules and theories in the field. Case reports are great to get first i…
Limitations of Case Reports
- Observing a relationship between an exposure and a disease in a case report does not mean that it is causal in nature. This is because of: 1. The absence of a control group that provides a benchmark or a point of reference against which we compare our results. A control group is important to eliminate the role of external factors which can interfere with the relationship between exposure and disease 2. Unmeasured Confounding caused by variabl…
Real-World Examples of Case Reports
- Example 1: Normal plasma cholesterol in an 88-year-old man who eats 25 eggs a day
This is the case of an old man with Alzheimer’s disease who has been eating 20-30 eggs every day for almost 15 years. [Source] The man had an LDL-cholesterol level of only 142 mg/dL (3.68 mmol/L) and no significant clinical atherosclerosis (deposition of cholesterol in arterial walls)! His body adapted by reducing the intestinal absorpti… - Example 2: Recovery from the passage of an iron bar through the head
This is an interesting case of a construction foreman named Phineas Gage. [Source] In 1848, due to an explosion at work, an iron bar passed through his head destroying a large portion of his brain’s frontal lobe. He survived the event and the injury only affected 1 thing: His personality! After the accident, Gage became profane, rough and di…
References
- Sayre JW, Toklu HZ, Ye F, Mazza J, Yale S. Case Reports, Case Series – From Clinical Practice to Evidence-Based Medicine in Graduate Medical Education. Cureus. 2017;9(8):e1546. Published 2017 Aug 7...
- Nissen T, Wynn R. The clinical case report: a review of its merits and limitations. BMC Res Notes. 2014;7:264. Published 2014 Apr 23. doi:10.1186/1756-0500-7-264.
Further Reading
Popular Posts:
- 1. mayflower medical group patient portal
- 2. david kowalski patient portal
- 3. agmg patient portal
- 4. wnc family medical center patient portal
- 5. umc university hospital and clinics patient portal
- 6. fmcod patient portal login
- 7. patient portal goshen family practice
- 8. mdi intellechart ophtalmology patient portal
- 9. austin reagonal clinic patient portal
- 10. st thomas medical gallatin tn patient portal