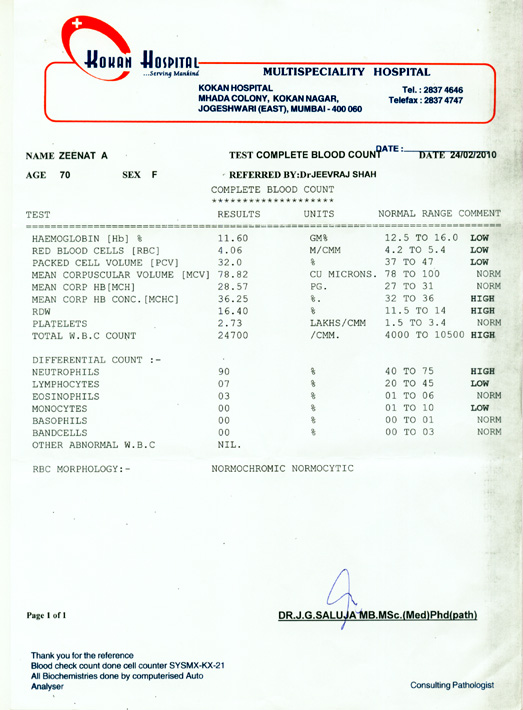
Babesiosis Case Report Form
3 hours ago · Babesiosis with high-grade parasitemia is life-threatening, especially in asplenic hosts. We report an asplenic patient with parasitemia >50% who was successfully treated with prompt red blood cell apheresis and triple therapy with clindamycin + azithromycin + atovaquone. >> Go To The Portal
Abstract A case of babesiosis in an asplenic individual is reported. A course characterized by fever, haemolysis, hepatitis, depressed mental status and non-cardiac pulmonary oedema was observed.
Full Answer
What antibiotics are used for asplenic patients with babesiosis?
What is a babesiosis tick?
Does quinine help with babesiosis?
Is RBC exchange good for parasitemia?
Is Babesiosis with high grade parasitemia life threatening?
Is clindamycin a good treatment for babesiosis?
See more
About this website
What antibiotics are used for asplenic patients with babesiosis?
In this case report, we describe a case of an asplenic patient who survived severe babesiosis with parasitemia >50% receiving an induction regimen combining clindamycin, azithromycin, and atovaquone, in addition to RBC exchange, followed by azithromycin and atovaquone for maintenance therapy.
What is a babesiosis tick?
Babesiosis, a tickborne illness transmitted by the Ixodes scapularis tick, has been increasing dramatically in incidence in the past decade, especially in the New England area [ 1 ].
Does quinine help with babesiosis?
Based on early animal model studies, we suggest that the efficacy of quinine in babesiosis treatment might be limited. As demonstrated by Rowin et al., quinine alone was not associated with parasitemia clearance in a hamster model. When compared with untreated controls (peak parasitemia levels 53%), the combination of clindamycin + quinine (peak 22%), compared with clindamycin alone (peak 27%), was only slightly better at reducing peak parasitemia levels, while parasitemia clearance after therapy ended in both treatment arms was complete [ 9 ]. Similarly, in another animal study comparing quinine, azithromycin, and quinine + azithromycin, quinine yielded the least antiparasitic effect compared with the other treatment group; when comparing the quinine + azithromycin group with the azithromycin only group, the parasitemia level was comparable at the end of the treatment course [ 10 ]. In light of this, we believe that the role of quinine in the recommended clindamycin + quinine combination adds considerable potential toxicity for minimal therapeutic gain. Thus, we would argue that atovaquone + azithromycin in place of quinine might be more efficacious against severe parasitemia with babesia, in addition to its superior side effect profile. Accumulating evidence from in vitro and animal models also suggests that agents including tafenoquine and clofazimine are potential candidates for severe babesiosis treatment [ 11–13 ]. Furthermore, we stress the critical importance of prompt RBC exchange in severe babesiosis to promptly decrease the parasitemia burden and halt further end-organ damage in the early phase of fulminant, life-threatening disease.
Is RBC exchange good for parasitemia?
[ 5 ]). However, there is no high-quality evidence to fully evaluate the utility of RBC exchange [ 2 ]. Based on a large case series of 34 cases, in which 7 patients underwent RBC exchange, a parasitemia level >10% was associated with the decision to perform RBC exchange, an arbitrary threshold unsupported by high-quality evidence. Clinicians would still need to be vigilant about post-RBC exchange parasitemia rebound [ 6 ]. Further clinical trials are warranted to fully evaluate the role of RBC exchange. While clindamycin and quinine are still recommended in the IDSA guideline [ 2 ], a growing body of evidence suggests that a quinine-free regimen may work just as well in babesiosis [ 4 ], even in severe cases [ 5, 7 ]. The rapid clinical improvement with RBC exchange in conjunction with this quinine-free regimen in this extraordinary level of parasitemia extends these observations.
Is Babesiosis with high grade parasitemia life threatening?
Babesiosis with high-grade parasitemia is life-threatening, especially in asplenic hosts. We report an asplenic patient with parasitemia >50% who was successfully treated with prompt red blood cell apheresis and triple therapy with clindamycin + azithromycin + atovaquone. This regimen may be an alternative to poorly tolerated clindamycin + quinine in severe cases.
Is clindamycin a good treatment for babesiosis?
In summary, our report demonstrates that the combination of clindamycin, azithromycin, and atovaquone together with prompt RBC exchange is efficacious in life-threatening babesiosis. We advocate further clinical trials to compare clindamycin + quinine and clindamycin + azithromycin + atovaquone to provide better evidence supporting this quinine-free regimen in severe babesiosis. In addition, new agents such as tafenoquine and clofazimine could be other alternatives to quinine in babesiosis treatment, and further studies are warranted.
What antibiotics are used for asplenic patients with babesiosis?
In this case report, we describe a case of an asplenic patient who survived severe babesiosis with parasitemia >50% receiving an induction regimen combining clindamycin, azithromycin, and atovaquone, in addition to RBC exchange, followed by azithromycin and atovaquone for maintenance therapy.
What is a babesiosis tick?
Babesiosis, a tickborne illness transmitted by the Ixodes scapularis tick, has been increasing dramatically in incidence in the past decade, especially in the New England area [ 1 ].
Does quinine help with babesiosis?
Based on early animal model studies, we suggest that the efficacy of quinine in babesiosis treatment might be limited. As demonstrated by Rowin et al., quinine alone was not associated with parasitemia clearance in a hamster model. When compared with untreated controls (peak parasitemia levels 53%), the combination of clindamycin + quinine (peak 22%), compared with clindamycin alone (peak 27%), was only slightly better at reducing peak parasitemia levels, while parasitemia clearance after therapy ended in both treatment arms was complete [ 9 ]. Similarly, in another animal study comparing quinine, azithromycin, and quinine + azithromycin, quinine yielded the least antiparasitic effect compared with the other treatment group; when comparing the quinine + azithromycin group with the azithromycin only group, the parasitemia level was comparable at the end of the treatment course [ 10 ]. In light of this, we believe that the role of quinine in the recommended clindamycin + quinine combination adds considerable potential toxicity for minimal therapeutic gain. Thus, we would argue that atovaquone + azithromycin in place of quinine might be more efficacious against severe parasitemia with babesia, in addition to its superior side effect profile. Accumulating evidence from in vitro and animal models also suggests that agents including tafenoquine and clofazimine are potential candidates for severe babesiosis treatment [ 11–13 ]. Furthermore, we stress the critical importance of prompt RBC exchange in severe babesiosis to promptly decrease the parasitemia burden and halt further end-organ damage in the early phase of fulminant, life-threatening disease.
Is RBC exchange good for parasitemia?
[ 5 ]). However, there is no high-quality evidence to fully evaluate the utility of RBC exchange [ 2 ]. Based on a large case series of 34 cases, in which 7 patients underwent RBC exchange, a parasitemia level >10% was associated with the decision to perform RBC exchange, an arbitrary threshold unsupported by high-quality evidence. Clinicians would still need to be vigilant about post-RBC exchange parasitemia rebound [ 6 ]. Further clinical trials are warranted to fully evaluate the role of RBC exchange. While clindamycin and quinine are still recommended in the IDSA guideline [ 2 ], a growing body of evidence suggests that a quinine-free regimen may work just as well in babesiosis [ 4 ], even in severe cases [ 5, 7 ]. The rapid clinical improvement with RBC exchange in conjunction with this quinine-free regimen in this extraordinary level of parasitemia extends these observations.
Is Babesiosis with high grade parasitemia life threatening?
Babesiosis with high-grade parasitemia is life-threatening, especially in asplenic hosts. We report an asplenic patient with parasitemia >50% who was successfully treated with prompt red blood cell apheresis and triple therapy with clindamycin + azithromycin + atovaquone. This regimen may be an alternative to poorly tolerated clindamycin + quinine in severe cases.
Is clindamycin a good treatment for babesiosis?
In summary, our report demonstrates that the combination of clindamycin, azithromycin, and atovaquone together with prompt RBC exchange is efficacious in life-threatening babesiosis. We advocate further clinical trials to compare clindamycin + quinine and clindamycin + azithromycin + atovaquone to provide better evidence supporting this quinine-free regimen in severe babesiosis. In addition, new agents such as tafenoquine and clofazimine could be other alternatives to quinine in babesiosis treatment, and further studies are warranted.