Cytoxan Advanced Patient Information - Drugs.com
33 hours ago · Uses for Cytoxan. Cyclophosphamide is used to treat cancer of the ovaries, breast, blood and lymph system, and nerves (mainly in children). Cyclophosphamide is also used for retinoblastoma (a type of eye cancer mainly in children), multiple myeloma (cancer in the bone marrow), and … >> Go To The Portal
Precautions
What are some things I need to know or do while I take Cytoxan? 1 Tell all of your health care providers that you take Cytoxan (cyclophosphamide injection). 2 Avoid driving and doing other tasks or actions that call for you to be alert or have clear eyesight... 3 Very bad and sometimes deadly allergic side effects have rarely happened...
What are some things I need to know or do while taking Cytoxan?
If you are taking Cytoxan (cyclophosphamide injection) once a day, take it in the morning. Do not take it at night unless told to do so by your doctor. Pass urine often.
When should I take Cytoxan (cyclophosphamide) injection?
Use birth control that you can trust to prevent pregnancy while taking Cytoxan (cyclophosphamide injection) and for up to 12 months after Cytoxan (cyclophosphamide injection). If you get pregnant while taking Cytoxan (cyclophosphamide injection) or within 12 months after your last dose, call your doctor right away.
What happens if I get pregnant while taking Cytoxan (cyclophosphamide)?
Use of some vaccines with Cytoxan (cyclophosphamide injection) may either raise the chance of an infection or make the vaccine not work as well. You may bleed more easily. Be careful and avoid injury. Use a soft toothbrush and an electric razor. You may have more of a chance of getting an infection. Wash hands often.
What should I avoid while using Cytoxan (cyclophosphamide)?
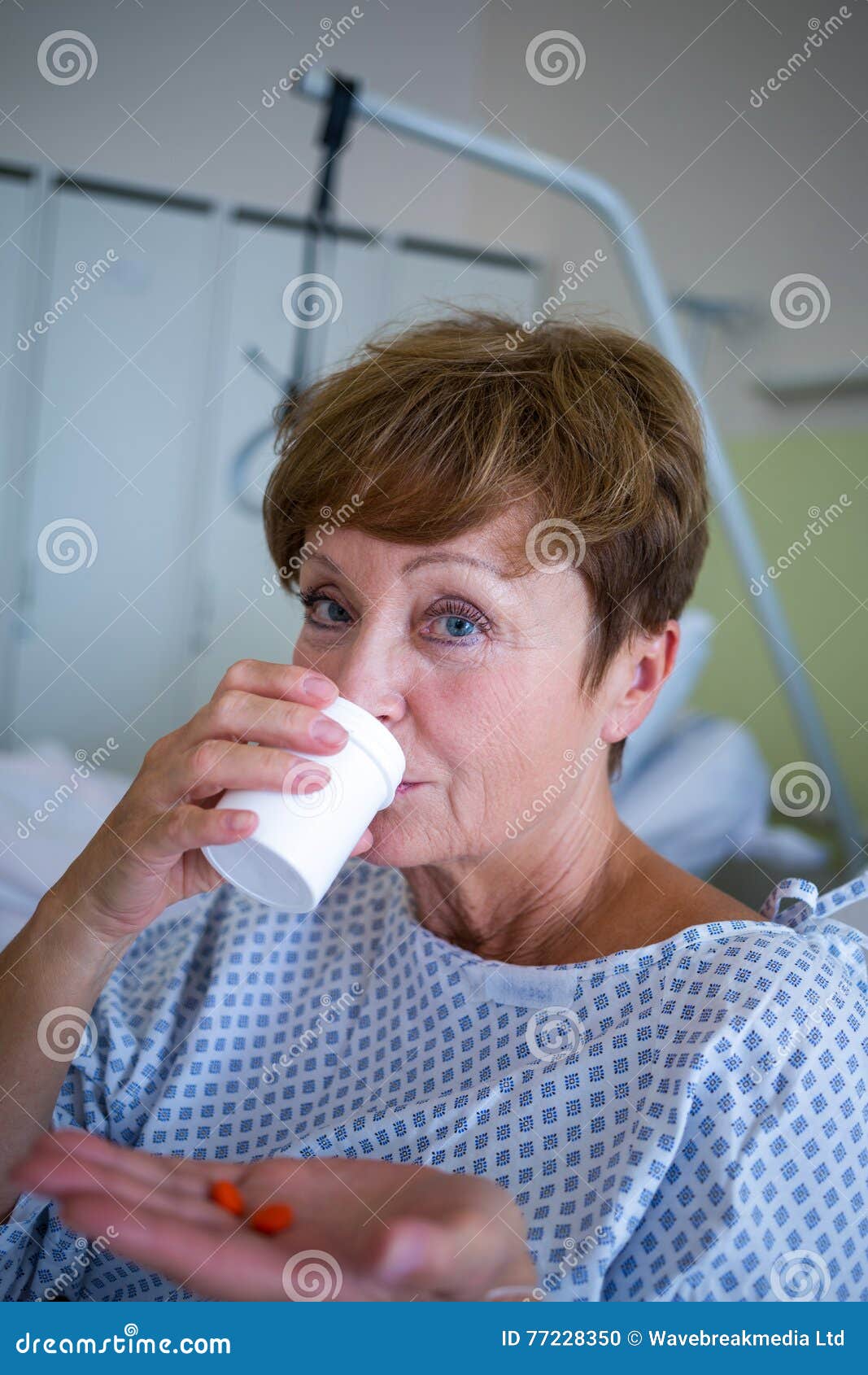
What should I watch when taking Cytoxan?
Check with your doctor immediately if you notice any unusual bleeding or bruising; black, tarry stools; blood in the urine or stools; or pinpoint red spots on your skin. Be careful when using a regular toothbrush, dental floss, or toothpick.
What should I monitor with cyclophosphamide?
Your doctor will order regular lab tests to check your response to cyclophosphamide and monitor for toxicity. Your blood counts – white blood cells, red blood cells and platelets – will be checked regularly. Your liver enzymes and urine will be checked regularly as well.
What instructions should be given to a patient taking Cytoxan?
Follow all instructions closely.It is given as an infusion into a vein over a period of time.If you are taking Cytoxan (cyclophosphamide injection) once a day, take it in the morning. ... Pass urine often. ... Drink lots of noncaffeine liquids unless told to drink less liquid by your doctor.More items...•
What are the side effects of taking Cytoxan?
Nausea, vomiting, loss of appetite, stomach ache, diarrhea, or darkening of the skin/nails may occur. Nausea and vomiting can be severe. In some cases, drug therapy may be necessary to prevent or relieve nausea and vomiting.
What is the most common side effect of cyclophosphamide?
Cyclophosphamide often causes nausea, vomiting, and loss of appetite. However, it is very important that you continue to use the medicine even if you begin to feel ill. Do not stop taking this medicine without first checking with your doctor. Ask your doctor for ways to lessen these effects.
Does Cytoxan have a black box warning?
Black Box Warnings: The main toxic effect of cytarabine injection is BONE MARROW SUPPRESSION with leukopenia, thrombocytopenia, and anemia.
Why is hydration important with cyclophosphamide?
Patients receiving cyclophosphamide or ifosfamide chemotherapy require intravenous fluid hydration to prevent hemorrhagic cystitis.
Which of the following is a concern when using oral chemotherapy agents?
Hyperglycemia (high blood glucose) is also a common side effect of some oral chemotherapy agents. Other common side effects of oral chemotherapy include nausea and vomiting, diarrhea, mouth sores, and skin rash, so patients must be educated on how to manage these side effects as well.
How do you handle cyclophosphamide?
Safe handling of cyclophosphamidePrepare a clean area where the drug can be handled safely (away from areas where food is prepared, out of the reach of children, and out from under any air vents or fans).We suggest you or the caregiver wear gloves while handling this medicine.More items...
Does Cytoxan cause hypotension?
In 100 people receiving Cyclophosphamide, from 4 to 20 may have: Damage to the bone marrow (irreversible) which may cause infection, bleeding, may require transfusions. Allergic reaction which may cause rash, low blood pressure, wheezing, shortness of breath, swelling of the face or throat.
When do cyclophosphamide side effects start?
Nausea and vomiting: more common with larger doses, usually beginning 6-10 hours after therapy.
What is the indication of cyclophosphamide?
Cyclophosphamide is indicated for the treatment of: malignant lymphomas (Stages III and IV of the Ann Arbor staging system), Hodgkin’s disease, lymphocytic lymphoma (nodular or diffuse), mixed-cell type lymphoma, histiocytic lymphoma, Burkitt’s lymphoma. multiple myeloma.
Why is hydration important with cyclophosphamide?
Patients receiving cyclophosphamide or ifosfamide chemotherapy require intravenous fluid hydration to prevent hemorrhagic cystitis.
How do you manage the side effects of cyclophosphamide?
Nausea and vomiting can be severe. In some cases, drug therapy may be necessary to prevent or relieve nausea and vomiting. Changes in diet such as eating several small meals or limiting activity may help lessen some of these effects. If these effects persist or worsen, notify your doctor or pharmacist promptly.
Does cyclophosphamide cause neutropenia?
To avoid the interaction of the animal immune system, in vivo antibiotic pharmacodynamic studies often employ cyclophosphamide (CPM) to induce neutropenia.
When do you need mesna with cyclophosphamide?
Mesna should always be given with ifosfamide and high dose cyclophosphamide if hyperhydration is not being used.
What to do if you have questions about Cytoxan?
If you have any questions about Cytoxan (cyclophosphamide injection), please talk with your doctor, nurse, pharmacist, or other health care provider.
What are some things I need to know or do while I take Cytoxan?
Tell all of your health care providers that you take Cytoxan (cyclophosphamide injection). This includes your doctors, nurses, pharmacists, and dentists.
How is this medicine (Cytoxan) best taken?
Use Cytoxan (cyclophosphamide injection) as ordered by your doctor. Read all information given to you. Follow all instructions closely.
What are some side effects that I need to call my doctor about right away?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
What are some other side effects of Cytoxan?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
How do I store and/or throw out Cytoxan?
If you need to store Cytoxan (cyclophosphamide injection) at home, talk with your doctor, nurse, or pharmacist about how to store it.
How to avoid bleeding from Cytoxan?
You may bleed more easily. Be careful and avoid injury. Use a soft toothbrush and an electric razor.
What is the trade name for Cytoxan?
Other Trade Name: Neosar ®. Cyclophosphamide is the generic name for the trade name drug Cytoxan or Neosar. In some cases, health care professionals may use the trade name Cytoxan or Neosar when referring to the generic drug name cyclophosphamide. Drug Type: Cyclophosphamide is an anti-cancer ("antineoplastic" or "cytotoxic") chemotherapy drug.
How long after taking Cyclophosphamide can you void?
It is important to void (empty your bladder) frequently especially in the first 24 hours after taking cyclophosphamide. Report any pain or burning on urination to your health care provider.
How is cyclophosphamide given?
It is usually given through a vein by injection or infusion (intravenous, IV) or by mouth in tablet form, depending upon diagnosis. Cyclophosphamide is also approved to be given by a shot into a muscle (IM), into the abdominal lining (intraperitoneal, IP), or into the lining of the lung (intrapleural).
What are the side effects of cyclophosphamide?
Discoloration of the skin or nails. The following are less common side effects (occurring in 10-29%) for patients receiving cyclophosphamide: Loss of fertility: Your ability to conceive or father a child may be affected by cyclophosphamide.
Does cyclophosphamide cause low blood count?
There are many options to minimize or prevent the side effects of cyclophosphamide. The following side effects are common (occurring in greater than 30%) for patients taking cyclophosphamide: Low blood counts: Your white and red blood cells and platelets may temporarily decrease.
Can you take aspirin with cyclophosphamide?
Before starting cyclophosphamide treatment, make sure you tell your doctor about any other medications you are taking (including prescription, over-the-counter, vitamins, herbal remedies, etc.) Do not take aspirin or products containing aspirin unless your doctor specifically permits this.
Can you use the same drug for other problems?
Note: If a drug has been approved for one use, physicians may elect to use this same drug for other problems if they believe it may be helpful.
Most voted negative review
The horrible side effects from this medication were not fully described to me by the prescribing physician. I was made sterile by this drug and put into early menopause. It DID NOT have any effect on my pulmonary fibrosis. It was prescribed so that it would slow the progression and it didn't work. It made me tired. I lost my hair.
Shared reviews and ratings
I am new to this medication. Literally been talking for 2 days for Nephrotic Syndrome (the only treatment there is for me). I take 75mg daily but alternate months with another medication. I have had severe weakness, tiredness, full body aches, upset stomach, vomiting, bone pain.
IMPORTANT INFORMATION ABOUT USER-GENERATED CONTENT ON WEBMD
The opinions expressed in WebMD User-generated content areas like communities, reviews, ratings, or blogs are solely those of the User, who may or may not have medical or scientific training. These opinions do not represent the opinions of WebMD.
How often should blood glucose be checked for clozapine?
Fasting blood glucose should be checked at baseline, after one months’ treatment, then every 4–6 months in patients taking clozapine.45,22)Lipid profile should be measured at baseline, at 3 months and then yearly for patients taking antipsychotics; however for patients on clozapine it is advised that it is done 3 monthly in the first year. 45)
How long does it take to monitor myocarditis?
Routine monitoring for myocarditis is suggested for the first 4 weeks of clozapine, and in the presence of evidence consistent with myocarditis discontinuation of clozapine is advised. Investigation by cardiac imaging will give a measure of severity and need for intervention. 20)
What can increase clozapine levels?
22)The use of cytochrome P450 inhibitors, such as antifungals, oral contraceptives, fluvoxamine, cimetidine , erythromycin , ciprofloxacin and caffeine can increase clozapine level;49,52,53)similarly, carbamazepine, phenytoin and possibly other enzyme inducers like phenobarbitone and rifampicin may decrease plasma clozapine. Fluvoxamine can increase clozapine level considerably and there are reported fatalities. 49)There are differences in reports about other selective serotonin re-uptake inhibitors; both increase,54,55)and no effect49)have been reported.
How often should I monitor my weight for schizophrenia?
It is suggested that patients with schizophrenia should have physical health monitoring (including cardiovascular disease risk assessment) at least once per year. 45)Specifically there should be BMI and waist circumference measurements at baseline and 1 month, 3 monthly and then yearly. 22)Weight should be measured for all patients on antipsychotics at baseline, subsequently at frequent intervals during the first 3 months, at 3 months and then yearly. Patients taking clozapine require more frequent monitoring of these parameters: every 3 months for the first year, then yearly. 45)
Can you stop WBC monitoring after 6 months?
It has been reported that discontinuation of white blood cell (WBC) monitoring after 6 months of starting clozapine has similar mortality associated with other medications or even with life in general involving accidents. 13)Based on this, some authors suggest that in well informed patients wishing to stop monitoring, it may be justifiable. 13)However, late onset clozapine-induced agranulocytosis has been reported,14–16)which suggests the probable need for continued monitoring for reducing mortality.
Can clozapine cause tachycardia?
The side effects have been found as potential reasons for clozapine discontinuation, which include: neutropenia or agranulocytosis, thrombocytopenia, electrocardiogram (ECG) changes, QTc prolongation, tachycardia, atrial flutter, myocarditis, cardiomyopathy, fever, syncope, diabetes mellitus, diabetic ketoacidosis, diabetic hyperosmolar coma, neuroleptic malignant syndrome, ileus, liver enzyme elevation and seizure. 10)It may be highlighted that these side effects can be detected, prevented, minimized and treated,2)which may decrease serious consequences including fatalities.
What are the health problems associated with Clozapine?
Diabetes, hypercholesterolemia, hypertriglyceridemia and obesity have been reported to be significantly associated with clozapine.23,24)Including these specific abnormalities, metabolic syndrome with a reported range of 43.2% to 70.4% is also associated with clozapine;25–28)which is a known cause of increased cardiovascular events and mortality.29–31)
Where to report buprenorphine side effects?
Report side effects from buprenorphine, methadone, or other medicines to the FDA MedWatch program, using the information in the "Contact FDA" box at the bottom of this page.
How to keep a list of all your medications?
It is helpful to keep a list of all your current medicines in your wallet or another location where the list is easily retrieved. You can fill out and print a copy of My Medicine Record.
What is the purpose of a buprenorphine orientation?
As a routine part of orientation to buprenorphine or methadone treatment, educate patients about the risks of concomitant use of benzodiazepines, sedatives, other prescribed opioid analgesics, alcohol, and illicit drugs.
Why is it important to lock up your medicines?
It is important to lock up your medicines and to dispose of them properly to keep them from being taken accidentally by children or falling into the wrong hands.
When did the FDA warn about benzodiazepines?
This provides updated information to the FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning issued on August 31, 2016.
What is coordinated care?
Coordinating care to ensure other prescribers are aware of the patient’s buprenorphine or methadone treatment.
What is the treatment for colorectal cancer?
Bevacizumab is a humanized monoclonal antibody that targets VEGF, thus preventing the ligand VEGF from binding to the endothelial cell VEGFR1 and 2. Bevacizumab is indicated for first- and second-line treatment of patients with advanced colorectal cancer in combination with fluorouracil (5-FU)-based chemotherapy given every 2 weeks in combination with bolus IFL (irinotecan [Camptosar], 5-FU, leucovorin) for a bevacizumab dose of 5 mg/kg IV, and with FOLFOX (leucovorin, infusional 5-FU, and oxaliplatin [Eloxatin]) for a bevacizumab dose of 10 mg/kg IV. It is also approved for the first-line treatment of patients with unresectable, locally advanced, recurrent or metastatic non–small-cell lung cancer (NSCLC) in combination with paclitaxel and carboplatin at a dose of 15 mg/kg IV given every 3 weeks.
What is the FDA approved treatment for solid tumors?
Three antiangiogenesis agents that are VEGF-targeted agents have been FDA-approved in the treatment of solid tumors: bevacizumab (Avastin), sunitinib (Sutent), and sorafenib (Nexavar).
Is SN-38 higher in chemotherapy?
Drug interaction studies showed that the concentration of the irinotecan active metabolite SN-38 was 33% higher in patients receiving bevacizumab plus IFL compared to chemotherapy alone, perhaps explaining the higher incidence of grade 3/4 diarrhea and neutropenia in the combination arm. [2]
Can you take sorafenib with chemotherapy?
Caution should be used when combining sorafenib with chemotherapy . Irinotecan is eliminated by the UGT1A1 pathway, and coadministration resulted in a 67% to 120% increase in the area under the concentration-time curve (AUC) of the active metabolite SN-38 causing potentially severe irinotecan toxicity; combination with doxorubicin resulted in a 21% increase in the AUC of doxorubicin, potentially increasing doxorubicin toxicity; and lastly, combination with docetaxel also raises serum docetaxel concentrations. Finally, in Japanese patients, drug mean steady-state AUC was 45% lower, so drug dose may need to be increased. [5]
What happens when you sedate a patient?
Sedate the patient the spasms and spasticity are decreased.
Should muscle relaxants be tapered?
Muscle relaxants should be tapered, rather than stopped abruptly, to avoid rebound spasms.
Does cyclobenzaprine affect the CNS?
Concomitant use of cyclobenzaprine and alcohol and other CNS depressants may intensify CNS depressant effects of the drug.
How old do you have to be to take cetirizine?
Adults and children 6 years and older can take cetirizine capsules and tablets.
How much does cetirizine cost?
You typically take it just once per day, and it begins to work quickly. It’s inexpensive, too — usually less than $1 per day for brand-name versions (Zyrtec, Aller-Tec, and Alleroff), and even less for generic products.
Does cetirizine help with hives?
Allergies usually affect your nose, sinuses, throat, and other areas of your upper respiratory system. Cetirizine also helps relieve hives. Hives are itchy, raised rashes on the skin. They often occur with food or medication allergies.
Is cetirizine safe for pregnant women?
Talk to your doctor or healthcare provider before you take cetirizine if you are pregnant or planning to become pregnant, or if you’re breastfeeding. Taking cetirizine is generally safe during pregnancy.
Is cetirizine safe to take?
Generally, cetirizine is a safe and effective drug, but you should be aware of certain warnings and precautions when taking this drug. Learn how this drug works, what it’s used for, and how to take it safely.
Can you drink alcohol while taking cetirizine?
For example, avoid consuming alcoholic drinks while you take cetirizine. Doing so may be dangerous. Mixing cetirizine with alcohol can cause drowsiness or make you less alert. If you take any type of tranquilizer, sedative, or sleep aid, make sure to mention this to your doctor before you use cetirizine.
Can you take cetirizine with kidney disease?
If you have liver or kidney disease, ask your doctor about taking cetirizine. If your doctor feels it’s safe for you to take, they may recommend taking less than the typical dosage.