Case report: patient's perspective on acute diabetic …
31 hours ago Case report: patient's perspective on acute diabetic neuropathy. C. S. Kargel, M. Godwin, and D. Alexander. ... LaMoreaux L, Garofalo E. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998 Dec 2; 280 (21):1831–1836. [Google Scholar] >> Go To The Portal
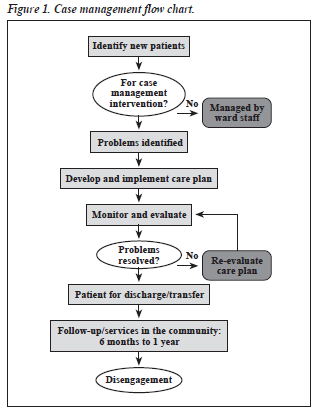
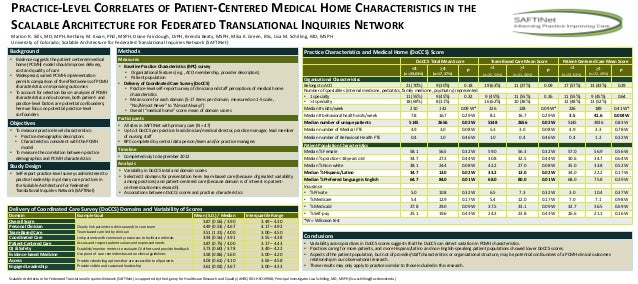
Some journals follow a case report format that includes the patient’s perspective. If authors had obtained informed consent from the patients at the time of commencement of the study, the patients might agree to contribute their perspective to the case report.
Full Answer
How do you write a patient perspective in a case report?
The patient should share their perspective on the treatment (s) they received in one to two paragraphs. It is often best to ask for informed consent and the patient’s perspective before you begin writing your case report.
What is the purpose of a patient case report?
Patient case reports are valuable resources of new and unusual information that may lead to vital research. Patient case reports are valuable resources of new and unusual information that may lead to vital research. How to write a patient case report
What are the five sections of a patient case report?
Summary: The format of a patient case report encompasses the following five sections: an abstract, an introduction and objective that contain a literature review, a description of the case report, a discussion that includes a detailed explanation of the literature review, a summary of the case, and a conclusion.
What information should be included in a patient perspective?
Patient Perspective – The patient should share their perspective on the treatment (s) they received. Informed Consent – The patient should give informed consent. (Provide if requested.) The CARE checklist (and writing outline) have been translated into multiple languages.
What does patient perspective mean?
“Patient perspective” is the patient's experience of PH and its impact on him/her and caregivers, including symptomatic, intellectual, psychosocial, spiritual and goal-oriented dimensions of the disease and its treatment.
How do you write a case report for a patient?
III. Patient case presentationDescribe the case in a narrative form.Provide patient demographics (age, sex, height, weight, race, occupation).Avoid patient identifiers (date of birth, initials).Describe the patient's complaint.List the patient's present illness.List the patient's medical history.More items...•
Can a case report be prospective?
5 Case reports/case series are usually retrospective although can occasionally be prospective, such as the Herrick's first case report of sickle cell disease. 1 Case reports/series can also define their subject by exposure or outcome (analogous to a cohort study and case–control study).
Why is it important to understand patients perspective?
Identifying patients' standpoints on their health condition and treatments offers an opportunity for critical discussion of differences of opinions and promotes communication exchange and agreement about the appropriate course of action.
How do you introduce a patient in a case study?
First, we describe the complaint that brought the patient to us. It is often useful to use the patient's own words. Next, we introduce the important information that we obtained from our history-taking. We don't need to include every detail – just the information that helped us to settle on our diagnosis.
What should a patient case study include?
Case: This section provides the details of the case in the following order:Patient description.Case history.Physical examination results.Results of pathological tests and other investigations.Treatment plan.Expected outcome of the treatment plan.Actual outcome.
Do you need patient consent for a case report?
Although not technically required, especially if the case report does not include any identifying information, some journals require informed consent for all case reports before publishing. The CARE guidelines recommend obtaining informed consent AND the patient's perspective on the treatment/outcome (if possible).
What are the characteristics of a good case report?
Case reports should be short and focused, with a limited number of figures and references. There are usually a restricted number of authors. The structure of a case report usually comprises a short unstructured (or no) abstract, brief (or no) introduction, report of the case, and discussion [Table 1].
How do you analyze a case study sample?
Writing a Case Study AnalysisRead and Examine the Case Thoroughly. Take notes, highlight relevant facts, underline key problems.Focus Your Analysis. Identify two to five key problems. ... Uncover Possible Solutions/Changes Needed. ... Select the Best Solution.
How do you elicit patient perspective?
Tips for Clinicians: Eliciting the Patient's PerspectiveWhat do you think is wrong?What do you think has caused the problem?What are you afraid this might be?What have you done to relieve the problem? ... Have you seen other health care professionals?What do you think would help you?More items...
Why are different perspectives important in healthcare?
While diverse perspectives and approaches to care are important, if they are not managed appropriately, they can cause misunderstandings, bias decision making, and get in the way of the best care.
What is the importance of understanding our patient's perception or concept of health and illness?
Acquiring a better awareness of a patient's health beliefs may help healthcare providers identify gaps between their own and the patient's understanding of his or her health situation. Consequently, this may lead to treatment choices more acceptable to the patient's expectations and needs.
How do you start a report?
The first section you start writing in your report is always a summary or introduction. This should stretch across just one or two pages to give your reader a brief glimpse into what your results or findings are.
What is the format for report writing?
Report writing is a formal style of writing elaborately on a topic. The tone of a report and report writing format is always formal. The important section to focus on is the target audience. For example – report writing about a school event, report writing about a business case, etc.
How do you write a nursing patient report?
How to write a nursing progress noteGather subjective evidence. After you record the date, time and both you and your patient's name, begin your nursing progress note by requesting information from the patient. ... Record objective information. ... Record your assessment. ... Detail a care plan. ... Include your interventions.
How do you write a client case history?
How to Write a Marketing Case Study that Lands You More ClientsDefine the type of clients you want to attract. ... Gather information and data points. ... Outline your case study. ... Be Human. ... Provide actionable advice. ... Write Clearly and Succinctly (Avoid industry jargon!) ... Publish and promote.
What is patient perspective?
What is "the patient perspective" in patient engagement programs? Implicit logics and parallels to feminist theories. Public and patient involvement (PPI) in health care may refer to many different processes, ranging from participating in decision-making about one's own care to participating in health services research, health policy development, ...
What is PPI in healthcare?
Public and patient involvement (PPI) in health care may refer to many different processes, ranging from participating in decision-making about one's own care to participating in health services research, health policy development, or organizational reforms.
What to ask for in a case report?
It is often best to ask for informed consent and the patient’s perspective before you begin writing your case report. Appendices (If indicated). Submission to a scientific journal. Follow author guidelines and journal submission requirements when writing and submitting your case report to a scientific journal.
Do you need informed consent for a journal?
The patient should provide informed consent (including a patient perspective) and the author should provide this information if requested. Some journals have consent forms which must be used regardless of informed consents you have obtained. Rarely, additional approval (e.g., IRB or ethics commission) may be needed.
What is a case report?
One of the best ways to tackle any piece of scholarly work is to seek to understand its purpose. A case report is most useful when it. Describes a new disease. Describes rare manifestations of a known illness. Clarifies the mechanism of an illness. Outlines adverse or beneficial side effects of a treatment course.
Why is it important to adhere to the case report format prescribed by the journal?
It is also important to obtain required patient consent and to follow the guidelines specified by the respective institutional review board. Drafting case reports. Leave a comment.
Why is a case report important?
Case reports have been described as being inferior and the weakest level of clinical evidence. While it is true that a journal case report cannot supersede the power of a clinical trial for the evidence based data it generates, the case report still has an important role to play in the medical literature. Case reports serve the function of proposing new hypotheses and sharing clinical observations that can then become the focus of a larger scientific study. Writing a report about a rare disease may be the primary way to publicize it, as small patient numbers would make it unlikely that such a disease would ever be part of a clinical trial or other research investigation. Learning how to write a case report is a skill that early stage researchers may need to acquire. The format of the case report is specific to the requirements of the target journal that, so it is important that the authors first consult the chosen journal’s instructions for authors before starting to draft the article.
What should authors refer to in case reports?
Authors should also refer to the more recent case reports published in the journal. This will help authors get an idea about the style that is expected and the types of case reports the journal tends to publish. Once that has been done, the next logical step is to focus on drafting the case report sections.
Why do you write a title after a case report?
Authors may choose to write the title after the rest of the report to ensure it reflects the tone of the predominant issue in the case report.
How many references should be included in a case report?
Extensive literature review should be avoided. In most cases, journals limit the references associated with a case report to no more than 15 papers.
What is the purpose of writing a case report?
The most important thing to bear in mind when it comes to how to write a case report is the purpose of writing one – which is to raise awareness about any unusual cases seen during the course of one’s medical practice. It is important to adhere to the case report format prescribed by the journal.
Why is it important to write a case report?
the reason you would go to the trouble of writing one, is that the case is sufficiently unique, rare or interesting such that other medical professionals will learn something from it.
What should I do after writing a case report?
Once you have written a draft of the case report, you should seek feedback on your writing, from experts in the field if possible, or from those who have written case reports before .
What is required to submit a case report?
Journals often have specific requirements for publishing case reports, which could include a requirement for informed consent, a letter or statement from the IRB and other things.
Is it free to publish a case report?
Be aware that it may not be free to publish your case report. Many journals charge publication fees. Of note, many open access journals charge author fees of thousands of dollars. Other journals have smaller page charges (i.e. $60 per page), and still others will publish for free, with an "open access option".
Do you need informed consent for a case report?
Although not technically required, especially if the case report does not include any identifying information, some journals require informed consent for all case reports. The CARE guidelines recommend obtaining informed consent AND the patient's perspective on the treatment/outcome (if possible).
Do journals have informed consent?
Journals may have their own informed consent form that they would like you to use, so please look for this when selecting a journal. Once you've identified the case, selected an appropriate journal (s), and considered informed consent, you can collect the required information to write the case report.
Does HIPAA apply to case reports?
First, you will be collecting protected health information, thus HIPAA applies to case reports. Spectrum Health has created a very helpful guidance document for case reports, which you can see here: Case Report Guidance - Spectrum Health. While this guidance document was created by Spectrum Health, the rules and regulations outlined could apply ...