How to write a patient case report - PubMed
13 hours ago Summary: The format of a patient case report encompasses the following five sections: an abstract, an introduction and objective that contain a literature review, a description of the case report, a discussion that includes a detailed explanation of the literature review, a summary of the case, and a conclusion. The abstract of a patient case report should succinctly include the four sections of the main text of the report. >> Go To The Portal
Writing Your Patient Case Study
- Work on Your Introduction Select a case. You have to identify your focus and scope for the study. ...
- Get to Know the Participants You can have one or multiple case participants. ...
- Perform Data Analyses Method Your results will depend on your interpretation of the raw data. ...
- Report the Case Study Results
- Patient description.
- Case history.
- Physical examination results.
- Results of pathological tests and other investigations.
- Treatment plan.
- Expected outcome of the treatment plan.
- Actual outcome.
What is the best way to write a case study?
There are usually eight sections in a case study:
- Synopsis/Executive Summary. Outline the purpose of the case study. ...
- Findings. This section is often divided into sub sections.
- Discussion. Summarise the major problem/s. ...
- Conclusion. Sum up the main points from the findings and discussion.
- Recommendations. ...
- Implementation. ...
- References. ...
- Appendices (if any)
How to write a more convincing case study?
Shapiro
- Take out the chaff in advance. “You don’t want students to spend too much time separating the wheat from the chaff. ...
- Work in layers and metaphors—subtly. “The best cases work on multiple levels. ...
- Encourage emotional engagement. “Case writing is an interesting literary form—it needs to be very engaging, but also educational. ...
How to identify a good case study?
The checklist with four phases to conduct a case study is given below:
- Foundation phase Philosophical consideration Inquiry techniques consideration Research logic consideration
- Prefield phase Decide Case study protocol
- Field phase Contact Interact
- Reporting phase Case study reporting
How to write a compelling customer case study?
You can use these steps to write a case study:
- Prepare for the case.
- Define your angle.
- Craft a narrative.
- Uncover solutions.
- Select a relatable solution.
- Include these sections.
What should I do after writing a case report?
Why is it important to write a case report?
What is required to submit a case report?
Does CHM have funding for publication?
Is it free to publish a case report?
Do you need informed consent for a case report?
Do journals have informed consent?
See more
About this website
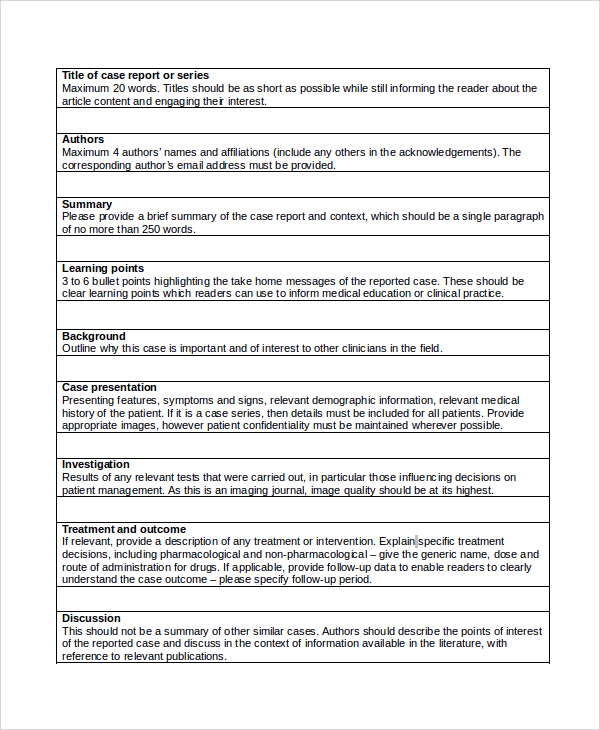
How do you write a patient case report?
III. Patient case presentationDescribe the case in a narrative form.Provide patient demographics (age, sex, height, weight, race, occupation).Avoid patient identifiers (date of birth, initials).Describe the patient's complaint.List the patient's present illness.List the patient's medical history.More items...•
How do you write a simple case study report?
How to Write a Case Study: The BasicsChoose the situation on which to write.Gather as much information as possible about the situation.Analyze all of the elements surrounding the situation.Determine the final solution implemented.Gather information about why the solution worked or did not work.
What is the format for writing a case study?
Your draft should contain at least 4 sections: an introduction; a body where you should include background information, an explanation of why you decided to do this case study, and a presentation of your main findings; a conclusion where you present data; and references.
What are some examples of case studies?
The Best Case Study ExamplesAdobe: Royal Bank of Scotland.BrightEdge: Stanley.LeadGnome: Host Analytics.Bitly: Vissla.Taboola: The Line.OutBrain: Lane Bryant.Google Analytics: Optimizely.LinkedIn: HubSpot.More items...•
How do you write a nursing case study report?
How to Write a Case Study Paper for NursingThe status of the patient. Demographic data. Medical History. ... The nursing assessment of the patient. Vital signs and test results. ... Current Care Plan and Recommendations. Details of the nursing care plan (including nursing goals and interventions)
What does a good case study look like?
Good case studies include key details that show how the customer got from A to B using the product—something you don't get with customer reviews. Don't make your reader work too hard to visualize the story. If you can use images and videos, use them!
Standard Case Report checklist and template for authors
I, [INSERT YOUR NAME IN FULL], the Author has the right to grant and does grant on behalf of all authors, an exclusive licence and/or a non-exclusive licence for contributions from authors who are: i) UK Crown employees; ii) where BMJ has agreed a CC-BY licence shall apply, and/or iii) in accordance with the relevant stated licence terms for US Federal Government Employees acting in the course ...
A guide to writing case reports for the Journal of Medical Case Reports ...
Case reports are a time-honored, important, integral, and accepted part of the medical literature. Both the Journal of Medical Case Reports and the Case Report section of BioMed Central Research Notes are committed to case report publication, and each have different criteria.Journal of Medical Case Reports was the world’s first international, PubMed-listed medical journal devoted to ...
Consent Form for Case Reports
Title: Consent Form for Case Reports Author: Wendy H Last modified by: Gilbreath, Jamie Created Date: 1/22/2018 1:45:00 PM Company: Portland Alternative Medicine
What should I do after writing a case report?
Once you have written a draft of the case report, you should seek feedback on your writing, from experts in the field if possible, or from those who have written case reports before .
Why is it important to write a case report?
the reason you would go to the trouble of writing one, is that the case is sufficiently unique, rare or interesting such that other medical professionals will learn something from it.
What is required to submit a case report?
Journals often have specific requirements for publishing case reports, which could include a requirement for informed consent, a letter or statement from the IRB and other things.
Does CHM have funding for publication?
It is best practice to check the journal's Info for Authors section or Author Center to determine what the cost is to publish. CHM does NOT have funds to support publication costs, so this is an important step if you do not want to pay out of pocket for publishing.
Is it free to publish a case report?
Be aware that it may not be free to publish your case report. Many journals charge publication fees. Of note, many open access journals charge author fees of thousands of dollars. Other journals have smaller page charges (i.e. $60 per page), and still others will publish for free, with an "open access option".
Do you need informed consent for a case report?
Although not technically required, especially if the case report does not include any identifying information, some journals require informed consent for all case reports. The CARE guidelines recommend obtaining informed consent AND the patient's perspective on the treatment/outcome (if possible).
Do journals have informed consent?
Journals may have their own informed consent form that they would like you to use, so please look for this when selecting a journal. Once you've identified the case, selected an appropriate journal (s), and considered informed consent, you can collect the required information to write the case report.
What is case study assessment?
In the UK, case studies are one of the most popular forms of assessment which are rolled out to students. Be it Nursing, economics, management, law or any other subject, students get a lot of case studies to complete.
What is the purpose of critical case studies?
There are two main purposes of writing these case studies, which are to challenge a universal assertion and investigating an area of interest. If the questions posed are the cause and effect questions, then writing these case studies is the best way to answer them.
What is pilot case study?
Pilot case studies. Also known as the exploratory nursing case studies, pilot case studies are condensed form of case studies. Before implementing an important investigation, nurses perform the analysis of such case studies. As per our nursing case study writing help experts, the main reason why students use this form of assessment is ...
Why do students need the guidance of experts?
The primary reason why students require the guidance of experts is that they are not able to deal with the pressing deadlines and secure top-notch grades in their work. For this, our case study writing help experts suggest them to pay attention to the intricacies in the structure of a case study.
Is there a shortcut to case study writing?
There’s no shortcut to extensive research when it comes to writing a case study in nursing. There has to be an inclusion of the vocabulary that is enriched with the important concepts of nursing. For that, you cannot fall short on information and relevant data.
Do you have to pay extra attention to case studies in nursing?
Therefore, you must pay extra attention to the structure of a nursing case study. Generally, the following is the structure that is adhered to by our experts while drafting a case study on a patient in nursing.
What is a patient case study?
Writing Your Patient Case Study. Since patient case studies are generally descriptive, they are under the a phenomenological principle. This means that subjectivity is entertained and allowed in research design. The medical scenarios are open to the researcher’s interpretation and input of insights.
What is a case study?
Case studies are a qualitative research method that offers a complete and in-depth look into some of the situations that baffled medical science. They document the cases that escape the ordinary in a hospital that has seen a manifold of plights. They serve as cautionary tales of the intricacy in dealing with human health.
How do case studies make a difference in the medical arena?
Patient case studies make a difference in the medical arena by reporting clinical interactions that can improve medical practices, suggest new health projects, as well as provide a new research direction. By looking at an event as it exists in the natural setting, case studies shed understanding on a complex medical phenomenon.
Why do medical practitioners use case studies?
Medical practitioners use case studies to examine a medical condition in the context of a research question. They perform research and analyses that adhere to the scientific method of investigation and abide by ethical research protocols. The following are case study samples and guides on case presentation.
Can you generalize a population using one case study?
You cannot generalize a population using one case study. However, multiple case study contains two or more cases under the point of interest can give you a replicated result. When the findings remain true for several cases under this research method, your case study’s results become more reliable.
Should you look into all possible explanations for a medical condition?
You should look into all of the possible explanations for the medical condition at hand. If a plight can be explained by more than one reason , then you have to look into the less obvious but similarly compelling explanations. Make your case study as informative as possible.
Is a clinical interaction report valid for generalization?
Since it documents stand-out clinical interactions where a single person or a few number of people are a party of, the findings may not be valid for generalization for a wider population.
Why do we need case studies?
Although they cannot be considered as guidelines, case studies are powerful material to share clinical experience and knowledge. You could write a case study to represent a typical or an unusual case presentation and share your successful program with your colleagues.
What is the purpose of the introduction in a report?
In the introduction, you give your readers an idea on the background of the case. Then discuss relevant cases and similar literature briefly. However, the most important aim of the introduction is driving your readers' attention to the purpose of your report. They should have a clear vision of your objectives.
Can you write a case study for Physioplus?
There are general guidelines for case studies but if you are writing for publication you have to be aware of the journal 's specific requirements. If you're writing a case study as part of Physioplus program assignment the following instructions will take you a step by step into the writing process.
What is a case report?
Case Report: A Beginner’s Guide with Examples. A case report is a descriptive study that documents an unusual clinical phenomenon in a single patient. It describes in details the patient’s history, signs, symptoms, test results, diagnosis, prognosis and treatment.
Why do we need case reports?
Case reports offer, in general a fast, easy and cheap way to report an unusual observation or a rare event in a clinical setting, as these have very small probability of being detected in an experimental study because of limitations on the number of patients that can be included.
Is a case report causal?
Observing a relationship between an exposure and a disease in a case report does not mean that it is causal in nature. The absence of a control group that provides a benchmark or a point of reference against which we compare our results. A control group is important to eliminate the role of external factors which can interfere with ...
Is a case report representative of the entire population?
So, results from a case report cannot be representative of the entire population.
What should I do after writing a case report?
Once you have written a draft of the case report, you should seek feedback on your writing, from experts in the field if possible, or from those who have written case reports before .
Why is it important to write a case report?
the reason you would go to the trouble of writing one, is that the case is sufficiently unique, rare or interesting such that other medical professionals will learn something from it.
What is required to submit a case report?
Journals often have specific requirements for publishing case reports, which could include a requirement for informed consent, a letter or statement from the IRB and other things.
Does CHM have funding for publication?
It is best practice to check the journal's Info for Authors section or Author Center to determine what the cost is to publish. CHM does NOT have funds to support publication costs, so this is an important step if you do not want to pay out of pocket for publishing.
Is it free to publish a case report?
Be aware that it may not be free to publish your case report. Many journals charge publication fees. Of note, many open access journals charge author fees of thousands of dollars. Other journals have smaller page charges (i.e. $60 per page), and still others will publish for free, with an "open access option".
Do you need informed consent for a case report?
Although not technically required, especially if the case report does not include any identifying information, some journals require informed consent for all case reports. The CARE guidelines recommend obtaining informed consent AND the patient's perspective on the treatment/outcome (if possible).
Do journals have informed consent?
Journals may have their own informed consent form that they would like you to use, so please look for this when selecting a journal. Once you've identified the case, selected an appropriate journal (s), and considered informed consent, you can collect the required information to write the case report.

Abstract
Format of The Patient Case Report
- Case reports should encompass the following five sections: an abstract, an introduction and objective with a literature review, a description of the case report, a discussion that includes a detailed explanation of the literature review, a summary of the case, and a conclusion.6,7 Supplementary parts such as tables, figures, graphs, and illustratio...
Summary
- Patient case reports are valuable resources of new and unusual information that may lead to vital research and advances in clinical practice that improve patient outcomes. Case reports should contain an abstract and four sections—an introduction, case presentation, discussion, and conclusion. The introduction provides the subject, purpose, and merit of the case report and the strategy used for the literature review. The patient case presentati…
Conclusion
- Patient case reports are valuable resources of new and unusual information that may lead to vital research.
Appendix A—Criteria For Publishable Case Reports
- Publishable patient case reports include cases that: 1. Advance medical science and spawn research; 2. Describe rare, perplexing, or novel diagnostic features of a disease state; 3. Report therapeutic challenges, controversies, or dilemmas; 4. Describe a new surgical procedure; 5. Report how a drug can enhance a surgical procedure; 6. Teach humanistic lessons to the health care professional; 7. Review a unique job description of a health care professio…
Popular Posts:
- 1. patient portal, power2practice
- 2. university patient portal colorado
- 3. tideland patient portal
- 4. penn state health st joes patient portal
- 5. sarasota retina institute patient portal dr levy
- 6. patient portal my settings tab
- 7. ovation fertility patient portal
- 8. capital health obgyn patient portal
- 9. mhpc patient portal
- 10. pediatrics of dalton patient portal