PATIENT REGISTRY ANNUAL DATA REPORT - Cystic Fibrosis …
4 hours ago The CF Foundation Patient Registry collects information on the health status of people with cystic fibrosis who receive care in CF Foundation-accredited care centers and agree to participate in the Registry. This information … >> Go To The Portal
What is the patient registry for cystic fibrosis?
The Patient Registry is an invaluable tool for researchers conducting observational studies about people with CF in the U.S. About 50,000 individuals have been followed in the Registry since its inception in 1986; many of them have been included for over 20 years. 4 min read
Where is the CF Foundation annual data report based?
ABOUT THIS REPORT The Annual Data Report is based on data entered in the CF Foundation Patient Registry through our online portal, PortCF©. Data are entered by teams of dedicated health professionals in our nationwide network of more than 120 CF Foundation-accredited Care Centers. Inclusion Criteria
What data is included in the CF Foundation patient registry?
The CF Foundation Patient Registry (CFFPR) is composed of data collected via the CF Foundation Care Center Network, including participant demographic characteristics, routine clinical measurements, therapeutic history, hospitalizations, transplant, and vital status.
How many people with cystic fibrosis are diagnosed in 2020?
Symptoms Reported at CF Diagnosis All Individuals (%) Diagnosed in 2020 (%) Diagnosed in 2020 Age < 1 (%) Diagnosed in 2020 Age ≥ 1 (%) Number of Individuals (n) 31,411 708 522 186 Asymptomatic

When was the Cystic Fibrosis Foundation established?
The Cystic Fibrosis Foundation Patient Registry was established in the 1960s and has continually evolved to keep pace with changes in technology and regulations, as well as improvements in the treatment of cystic fibrosis (CF).
How many patients were seen at CF Foundation in 2012?
As reported by the care centers during the annual reaccreditation process, there were 1,875 patients who were seen at accredited care centers in 2012 who did not provide consent to participate in the registry. This represents 6.3% of patients seen at CF Foundation–accredited care centers in 2012.
What does CF stand for in medical terms?
Definition of abbreviations: CF = cystic fibrosis; MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-sensitive Staphylococcus aureus. An audit of Cystic Fibrosis Foundation Patient Registry (CFFPR) data suggests high accuracy and low missingness of most variables compared with the medical record.
How many people in the US have CF in 2012?
On the basis of the two methods of estimating the number of persons with CF in the United States using national birth and death data, we derived estimates of 33,292 and 34,327 individuals with CF in the United States in 2012, respectively. In 2012, the CFFPR contained 27,804 individuals. Thus, approximately 81–84% of persons with CF were captured in the CFFPR in 2012, the most recent year for which national birth and mortality data were available.
How is CFFPR data collected?
The CFFPR data are collected through a web-based portal, PortCF, which contains five electronic data capture forms: demographic, diagnosis, encounter, care episode, and annual review forms. All data are entered by staff at the care center programs from the data available in the medical record or in forms completed by patients or families. CFFPR questionnaires are available in the annual reports ( 2 ).
What is CF Foundation?
All individuals diagnosed with CF and associated disorders (CFTR-related metabolic syndrome and CFTR-related disorders) who are seen at CF Foundation–accredited care center programs and provide informed consent are eligible to participate in the CFFPR. The CF Foundation has developed and sustains a network of 121 accredited CF care centers (comprised of 121 pediatric care programs and 105 adult care programs) and 51 affiliate programs across the United States ( see additional information available in the online supplement). All accredited care programs are required to participate in the CFFPR. A portion of care center funding is based on the number of patients enrolled in the CFFPR and the completeness of their records. Each program obtains institutional review board approval and written informed consent and assent, as appropriate, from participants and/or their legal guardians. The CF Foundation provides online user manuals, data entry guidelines, training sessions, and user support for care center staff who enter data into the CFFPR. The CF Foundation serves as the coordinating center for data collection and data analysis.
When was CFFPR established?
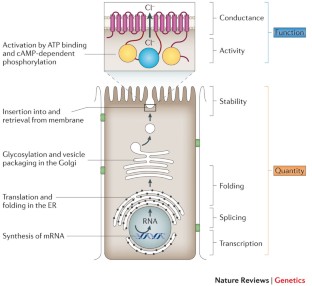
The CFFPR was established in the 1960s to collect information on patient demographics and survival ( 3 ). Regular updates have been made to comply with changes in regulations, improvements in data collection, and advances in new technology ( Figure 1) ( 4 ).
What is the CFF?
The US Cystic Fibrosis Foundation (CFF) began in 1955 with a mission to support the development of new drugs to fight the disease, improve the quality of life for those with cystic fibrosis (CF), and ultimately to find a cure for this disease.1 The CFF does this by supporting basic science and clinical research in CF, supporting the care of CF patients through accredited CF centres nationwide and advocating for CF patients at the state and national level. Recognising the critical role of data collection and measurement of outcomes to better understand the natural history of CF, the CFF created a patient registry in 1966, the CFF Patient Registry (CFFPR).2 The CFFPR has evolved over the years from a few demographic variables including vital status to a comprehensive database that gives healthcare providers, researchers, policy makers and change agents data to support epidemiological and clinical research as well as efforts to improve quality of care. The specific purpose of this commentary is to describe the CFFPR and primarily to focus on how the CFFPR and its associated tools are being used for quality improvement (QI) activities, with the hope that it may help CF healthcare teams in the USA who are not familiar with the registry's capabilities, CF providers outside the USA with registries at various stages of development, and others interested in how a patient registry has been used to improve care. The CFFPR contains detailed demographic and diagnostic data dating back to 1986 with current annual and encounter-based data on over 300 unique variables including outcomes (eg, microbiology, lung function and nutritional metrics, CF complications) and care processes (eg, hospitalisations, medications, surveillance measures) for each of its more than 27 000 participants in 2012; in all, there are over 46 000 unique individuals’ data in the registry.3 …
What is CF in Cyprus?
Background Specialized clinical care for cystic fibrosis (CF) in Cyprus, a small island country, has been implemented since the 1990s. However, only recently, a national CF patient registry has been established for the systematic recording of patients’ data. In this study, we aim to present data on the epidemiological, genotypic and phenotypic features of CF patients in the country from the most recent data collection in 2019, with particular emphasis on notable rare or unique cases. Results Overall, data from 52 patients are presented, 5 of whom have deceased and 13 have been lost to follow-up in previous years. The mean age at diagnosis was 7.2 ± 12.3 years, and the mean age of 34 alive patients by the end of 2019 was 22.6 ± 13.2 years. Patients most commonly presented at diagnosis with acute or persistent respiratory symptoms (46.2%), failure to thrive or malnutrition (40.4%), and dehydration or electrolyte imbalance (32.7%). Sweat chloride levels were diagnostic (above 60 mmol/L) in 81.8% of examined patients. The most common identified mutation was p.Phe508del (F508del) (45.2%), followed by p.Leu346Pro (L346P) (6.7%), a mutation detected solely in individuals of Cypriot descent. The mean BMI and FEV1 z-scores were 0.2 ± 1.3 and − 2.1 ± 1.7 across all age groups, respectively, whereas chronic Pseudomonas aeruginosa colonization was noted in 26.9% of patients. The majority of patients (74.5%) were eligible to receive at least one of the available CFTR modulator therapies. In 25% of patients we recovered rare or unique genotypic profiles, including the endemic p.Leu346Pro (L346P), the rare CFTR-dup2, the co-segregated c.4200_4201delTG/c.489 + 3A > G, and the polymorphism p.Ser877Ala. Conclusions CF patient registries are particularly important in small or isolated populations, such as in Cyprus, with rare or unique disease cases. Their operation is necessary for the optimization of clinical care provided to CF patients, enabling their majority to benefit from evolving advances in precision medicine.