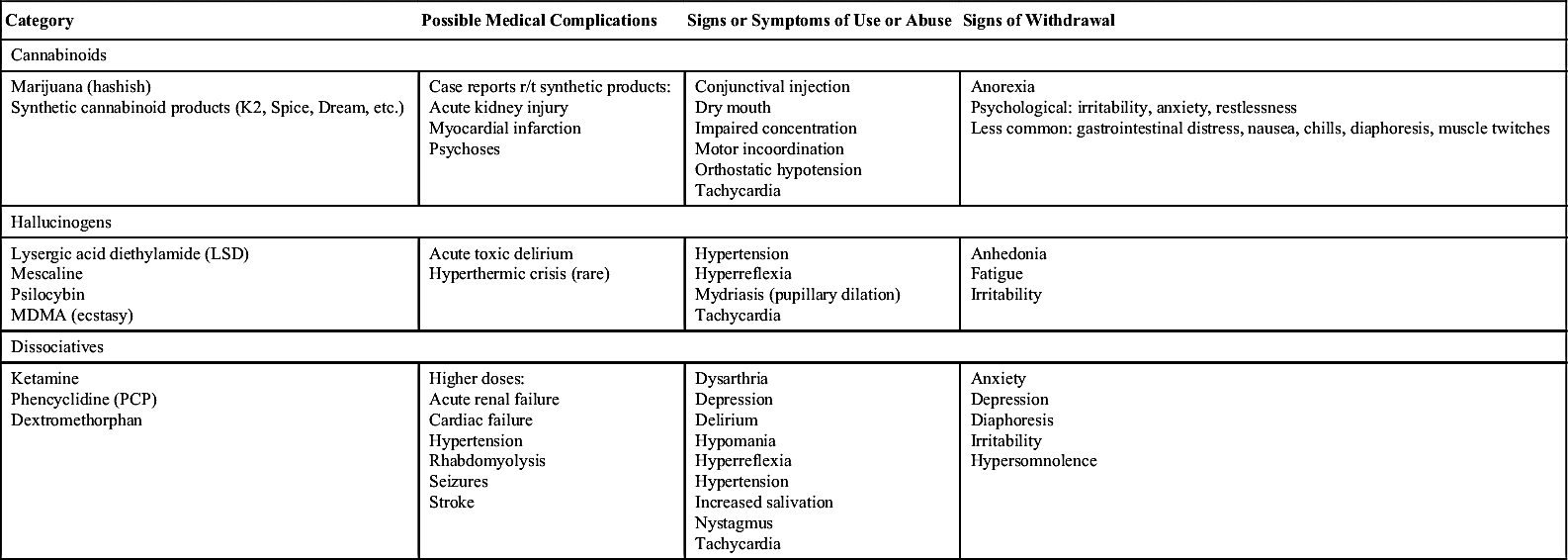
Controlled Substance Utilization Review and Evaluation …
8 hours ago The NC Controlled Substances Reporting System (CSRS) collects information on dispensed controlled substance prescriptions and makes this information available to prescribers and … >> Go To The Portal
This requirement means that unless an exemption exists in law, a physician must query the CURES database and run a Patient Activity Report (PAR) on each patient the first time a patient is prescribed, ordered, or administered a Schedule II-I V controlled substance.
Full Answer
What are the new reporting requirements for Controlled Substances?
Revised Reporting Requirements for Controlled Substances – The dispensing of a controlled substance must be reported to the Controlled Substance Utilization Review and Evaluation System (CURES) within one working day after the medication is released to the patient or the patient’s representative.
What is submission of controlled substance data?
Submission of Controlled Substance Data. California Health & Safety Code Section 11165(d) requires dispensing pharmacies, clinics, or other dispensers of Schedule II through IV controlled substances to provide specified dispensing information to the Department of Justice on a weekly basis in a format approved and accepted by the DOJ.
How long do you have to report controlled substance dispensing?
Previously, the deadline to report was seven days after dispensing. Further, this law requires reporting of Schedule V drugs, in addition to Schedules II, III, and IV. This requirement applies to pharmacists and prescribers who dispense controlled substances.
Are You required to register for controlled substance utilization review and evaluation?
Beginning on 1/1/22, all data reporters that currently submit prescription dispensation data to www.aaicures.com will be required to register Controlled Substance Utilization Review and Evaluation System | State of California - Department of Justice - Office of the Attorney General Skip to main content
What is a patient activity report?
WISHIN's Patient Activity Report (or PAR) provides a daily notification to payers (called the PAR-P) or providers/clinics (called the PAR-C) when their member/patient has had an emergency department (ED) or other hospital visit.
What information is displayed on the CURES activity report?
A CURES Patient Activity Report contains , as applicable, the following information: patient first name, patient last name, patient date of birth, patient gender, patient address, animal name, number of prescriptions, prescriber name, prescriber DEA number, prescriber address, pharmacy name, pharmacy license number, ...
What is controlled substance Utilization Review and Evaluation?
CURES (Controlled Substance Utilization Review and Evaluation System) is a database of Schedule II, Schedule III, Schedule IV and Schedule V controlled substance prescriptions dispensed in California serving the public health, regulatory oversight agencies, and law enforcement.
Who can run a CURES report?
While a physician can have a registered delegate request the CURES report, the report will go into the physician's dashboard on CURES so the physician can review the PAR prior to prescribing, ordering, administering, or furnishing. 4.
How far back does a CURES report go?
Patient Search – Prescribers & Dispensers For Prescriber, Dispenser, and Delegate users, CURES records can be searched up to 12 months using the date range option.
Is Adderall a controlled substance?
DRUG ABUSE AND DEPENDENCE ADDERALL® is a Schedule II controlled substance. Amphetamines have been extensively abused. Tolerance, extreme psychological dependence, and severe social disability have occurred.
Is Gabapentin a controlled substance?
by Drugs.com The anti-seizure medication gabapentin is not currently considered a narcotic or controlled substance by the federal government, but certain states have enacted legislation so that the medication is treated as one or monitored by the state's prescription drug monitoring program.
How do I access a cure report?
For assistance with CURES registration, access, or system use, contact the CURES helpdesk at CURES@doj.ca.gov or (916) 210-3187.
Is tramadol a controlled substance?
The Drug Enforcement Administration (DEA), the Centers for Disease Control and Prevention (CDC) and the Ultram® package insert indicate that tramadol is a controlled substance which contains an opioid.
Do pharmacists have to check CURES?
California licensed pharmacists must register for access to CURES 2.0 by July 1, 2016, or upon issuance of a Board of Pharmacy Pharmacist License, whichever occurs later.
Who can access CURES database?
Who has access to CURES information? As outlined in Health & Safety Code section 11165.1(a)(1)(A), prescribers authorized to prescribe, order, administer, furnish, or dispense Schedule II, III, or IV controlled substances, and pharmacists, may access CURES data for patient care purposes.
Is Ambien a controlled substance?
AMBIEN is a federally controlled substance (C-IV) because it can be abused or lead to dependence. Keep AMBIEN in a safe place to prevent misuse and abuse.
What is the purpose of the Prescription Safety Act and TN Together?
The Prescription Safety Act and TN Together Legislation represented a significant effort by the General Assembly to address the problem of prescription drug abuse. These Acts have significantly changed the regulations related to the CSMD.
Who is required to submit certain data to the CSMD?
Healthcare practitioners or persons under the supervision and control of the practitioners, pharmacists, or pharmacies who are legally authorized to dispense a schedule II, III, IV, or V controlled substance are required to submit certain data to the CSMD.
What does a supervisor do when a health care extender or delegate adds a supervisor?
The Supervisor will receive an email every time a Health Care Extender/Delegate or an APRN/PA adds that user as a supervisor to make the supervisor aware of the action. It is the responsibility of the supervisor to log in the CSMD and either approve or revoke the supervisory relationship. At any point in time, you no longer supervise a CSMD user in your account, log into the CSMD and “inactive/revoke” that relationship by logging in to the CSMD and navigating to “My Account”.
How often do you have to check for a new episode of treatment?
All healthcare practitioners are required to check before prescribing an opioid or benzodiazepine to a human patient as a new episode of treatment and every six (6) months thereafter when said controlled substance remains a part of the treatment. A new episode of treatment means a prescription for a controlled substance that has not been prescribed by that healthcare practitioner within the previous six (6) months . A new episode of treatment includes not only changes to specific drugs, but all changes to the strength of the drug prescribed, and the frequency with which the drug is taken.
How to register for TNCSMD?
Go to www.tncsmd.com and click on the word “register” to begin the registration process. Completion of the registration process will require specific identifying elements. Once registration is complete and approved, you will receive an email with your username and instructions to create a password. Medical Examiner role is only for State Medical Examiners.
What to do if you can't log in to CSMD?
If you are unable to log in, you may select Forgot/Reset Password and provide answers to the security questions you selected at registration or choose to have the system send a link to the email contained in your “My Account”/user profile in the CSMD. You will be able to create a new password using one of the two options provided. If you are unable to create a new password from those two options, then you will need to send an email to CSMD.ADMIN@tn.gov : or call 615-253-1305 for assistance.
Where is the button on the CSMD?
There is a button on the right side of the menu bar that allows you to move easily from the CSMD to the data collection site for dispensers. Information regarding Data Collection is below.
What is a DEA?
(a) (1) (A) (i) A health care practitioner authorized to prescribe, order, administer, furnish, or dispense Schedule II, Schedule III, or Schedule IV controlled substances pursuant to Section 11150 shall, before January 1, 2016, or upon receipt of a federal Drug Enforcement Administration (DEA) registration, whichever occurs later, submit an application developed by the Department of Justice to obtain approval to access information online regarding the controlled substance history of a patient that is stored on the Internet and maintained within the Department of Justice, and, upon approval, the department shall release to that practitioner the electronic history of controlled substances dispensed to an individual under his or her care based on data contained in the CURES Prescription Drug Monitoring Program (PDMP).
How to apply for PDMP in CA?
You can start the process by going to https://oag.ca.gov/cures click on the link PDMP Registration, then click on the link for Practitioner, and then follow the instructions to complete the application.
What is a controlled substance review?
review a patient’s controlled-substance prescription history to assess, identify, and prevent prescription drug abuse and
What drugs do 12th graders abuse?
In 2013, the “Monitoring the Future” survey found that among the top 10 substances abused by 12th-graders, 6 of them were pharmaceuticals (i.e., Adderall, Vicodin, OxyContin, cough medicine, tranquiliz- ers, and sedatives).
What is utilization review and evaluation system?
Utilization Review and Evaluation System: A Tool for Judicious Prescribing
What is the definition of drug diversion?
Drug diversion is when a drug leaves legitimate medical practice , such as a patient giving away or selling the medication.
Does the CURES bill require registrants to use the system?
Although the bill does not require CURES registrants to use the system, routinely checking CURES Patient Activ
Who can access electronic history of controlled substances?
As outlined in Health & Safety Code section 11165.1 (a) (1) (A), health care practitioners authorized to prescribe, order, administer, furnish, or dispense Schedule II, III, or IV controlled substances, and pharmacists, may access the electronic history of controlled substances dispensed to an individual under the practitioner or pharmacist’s care.
Who reports CURES?
Data contained in CURES is reported to CA DOJ by pharmacies, clinics and other dispensers. If you are a patient or prescriber with incorrect information on your CURES report, please notify the reporting pharmacy of the error. The reporting dispenser creates and owns the prescription records submitted. The Department of Justice is a custodian (and not editor) of these aggregated prescription records.
What is CURES database?
The CURES database also provides health care practitioners and pharmacists with a messaging capability that allows a message to be sent to another health care practitioner regarding a mutual patient from within the secure CURES environment.
What is the purpose of CURES data?
Additionally, pursuant to Health & Safety Code section 11165 (c) (2) (a), data obtained from CURES shall only be provided to appropriate state, local, and federal public agencies for disciplinary, civil, or criminal purposes and to other agencies or entities, as determined by the department, for the purpose of educating practitioners and others in lieu of disciplinary, civil, or criminal actions. Data may be provided to public or private entities, as approved by the California Department of Justice (CA DOJ), for educational, peer review, statistical, or research purposes, if patient information, including information that may identify the patient, is not compromised.
How often do you need to consult CURES database?
Effective October 2, 2018, with specified exceptions, health care practitioners authorized to prescribe, order, administer, or furnish a controlled substance shall consult the CURES database to review a patient's controlled substance history no earlier than 24 hours, or the previous business day , before prescribing a Schedule II, Schedule III, or Schedule IV controlled substance to the patient for the first time and at least once every 6 months thereafter if the substance remains part of the treatment of the patient. (Health and Safety Code section 11165.4 (a) (1) (A) (i))
What is the mandatory consultation requirement?
Pursuant to Health and Safety Code section 11165.4 (a), the mandatory consultation requirement requires health care practitioners to consult the CURES database to review a patient's controlled substance history under both of the following circumstances:
How to register a pharmacist in California?
Applicants must complete the online registration form and provide a valid email address, medical or pharmacist license number, and DEA registration certificate number ( when applicable).
What Schedules are required to report drugs?
Further, this law requires reporting of Schedule V drugs, in addition to Schedules II, III, and IV. This requirement applies to pharmacists and prescribers who dispense controlled substances.
When will California e-prescribing requirements be implemented?
Beginning January 1, 2022, all prescriptions issued by a licensed healthcare practitioner to a California pharmacy must be submitted electronically. For more information on this law and its requirements, please see the AB 2789 Bulletin.
When will the CURES system be implemented?
Assembly Bill (AB) 528 (Low, Chapter 677, Statutes of 2019) expands access and use of the Controlled Substance Utilization Review and Evaluation System (CURES) effective July 1, 2021. Please see the Joint Bulletin for an update on implementation of system enhancements for delegates and non-Drug Enforcement Agency (DEA) practitioners.
Can a California prescriber register with CURES?
Only licensed California prescribers and dispensers can register with CURES. ( NOTE: If you are with a law enforcement agency or regulatory board and need CURES access, please contact the CURES helpdesk at CURES@doj.ca.gov or (916) 210-3187). To register, licensed prescribers and dispensers will need:
What is the purpose of the California Prescription Drug Monitoring Program?
The mission of the California Prescription Drug Monitoring Program ( PDMP) is to eliminate pharmaceutical drug diversion in the state while promoting legitimate medical practice and quality patient care.
Who can provide data to the Department of Justice?
Data may be provided to public or private entities, as approved by the Department of Justice, for educational, peer review, statistical, or research purposes, provided that patient information, including any information that may identify the patient, is not compromised.
When was the California Triplicate Prescription Program created?
The California Triplicate Prescription Program (TPP) was created in 1939 , capturing Schedule II prescription information. CURES was initiated, operating in parallel with the TPP’s Automated Triplicate Prescription System (ATPS) to evaluate the comparative efficiencies between the two systems.