Common Formats | PSO
27 hours ago · Common Formats for Patient Safety Data. NQF coordinates the collection of input from stakeholders about “Common Formats” – a standardized method for healthcare providers and Patient Safety Organizations to collect and exchange information for any patient safety event. Read more. ACCESS DOCUMENTS - Common Formats. >> Go To The Portal
Technical Release Notes
The Technical Release Notes contain descriptions of changes that were made to the CFER-H V2.0. Changes include specificity on questions, answers, definitions, and summarizes changes to the Implementation Guide, Resources Workbook, and Flow Charts.
Supporting Documents
A users' guide and event descriptions for the Generic and event-specific categories are available for this version of Common Formats.
Technical Specifications
The technical specifications include the Implementation Guide, Resources Workbook, Data Dictionary, Data Submission Error Messages, Flow Charts, and CDA XML Samples that provide direction on how to implement and submit CFER-H V2.0 data.
Supplemental Information
The following event descriptions include the supplemental data elements that may be collected at the local level for additional analysis, and may be reported to PSOs but will NOT be accepted by the PSOPPC for national aggregation and analysis.
What is the Patient Safety and Quality Improvement Act?
299 b-21 to b-26, and the related Patient Safety and Quality Improvement Final Rule (Patient Safety Rule), 42 CFR part 3, published in the Federal Register on November 21, 2008, 73 FR 70731 -70814, provide for the formation of Patient Safety Organizations (PSOs), which collect, aggregate, and analyze confidential information regarding the quality and safety of health care delivery. The collection of patient safety work product allows for the aggregation of data that help to identify and address underlying causal factors of patient safety and quality issues.
What are the three settings of care that AHRQ maintains?
AHRQ previously developed and maintains Common Formats for three settings of care—acute care hospitals, skilled nursing facilities, and community pharmacies —for use by healthcare providers and PSOs.
What is CFER DS?
The CFER-DS is not designed for frontline incident reporting. It is intended to facilitate the collection and organization of a basic set of meaningful data about diagnostic safety events that can be used, aggregated and analyzed for learning and improvement.
Can a PSO use the common format?
Additionally, health care providers and other organizations not working with an AHRQ-listed PSO can use the Common Formats in their work to improve quality and safety; however, they cannot benefit from the federal confidentiality and privilege protections of the Patient Safety Act.
What is a CDA XML file?
The CDA XML file samples provide sample patient safety concerns and associated CDA XML file output for an Incident, Near Miss, and Unsafe Condition. Each sample contains all required data elements for each patient safety report, and conforms to the Community Pharmacy Common Formats.
What is CFER CP?
Common Formats for Event Reporting - Community Pharmacy Version 1.0 (CFER-CP V1.0) is one module in a series of Common Formats released by AHRQ. The module is primarily designed for use in the community pharmacy environment to gain enhanced understanding about the circumstances surrounding patient safety concerns in that setting. The framework applies a structured approach to the analysis of all community pharmacy events.
What is alternate data submission?
The Alternate Data Submission enables the submission of patient safety data in simple XML or DSV formatted files to the PSOPPC. The PSOPPC converts the XML or DSV files into the AHRQ Common Format required CDA XML format.
What is a glossary in pharmacy?
The glossary includes terms that have been defined to meet the purposes of, and to be consistent with, the conceptual model used to develop the Common Formats for Event Reporting - Community Pharmacy.
What is the Patient Safety and Quality Improvement Act?
The Patient Safety and Quality Improvement Act of 2005 (Patient Safety Act) provides for AHRQ to develop standardized reporting formats using common language and definitions (Common Formats) for reporting on health care quality and patient safety that will ensure that data collected by PSOs and other entities have comparable clinical meaning.
What is the purpose of CFER-DS?
In addition, the Common Formats are intended to enhance the reporting of information that is standardized both clinically and electronically. The CFER-DS is the first AHRQ Common Formats for Event Reporting that can be used across healthcare settings.
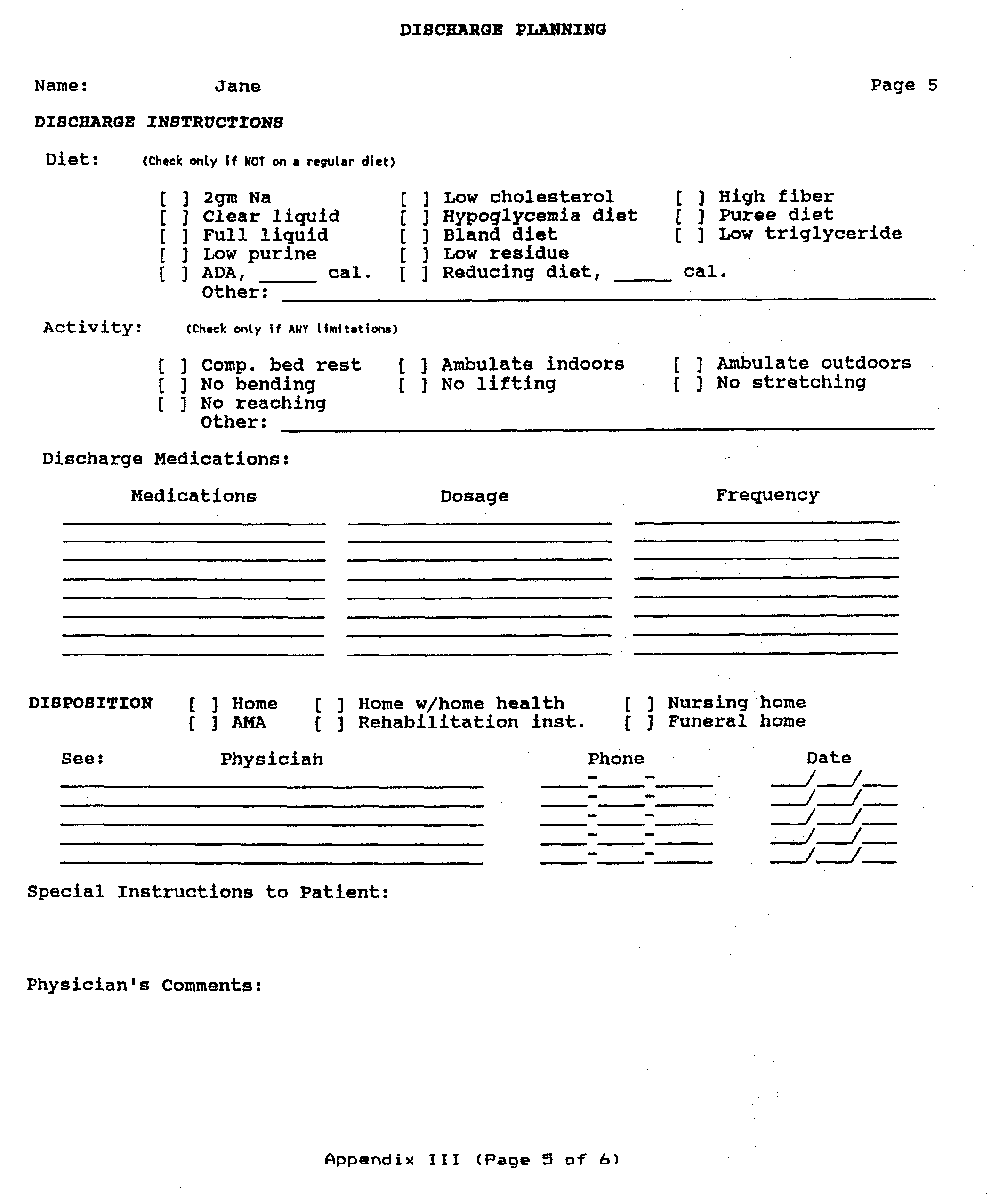
Background
Definition of Common Formats
- The term “Common Formats” is used to describe clinical definitions and technical requirements developed for the uniform collection and reporting of patient safety data, including all supporting material. The Common Formats are not intended to replace any current mandatory reporting system, collaborative/voluntary reporting system, research-related ...
Common Formats Development
- In anticipation of the need for Common Formats, AHRQ began their development in 2005 by creating an inventory of functioning private and public sector patient safety reporting systems. This inventory provides an evidence base that informs construction of the Common Formats. The inventory now numbers 69 and includes many systems from the private sector, including promin…
Mon Formats Version 1.1—Technical Specifications Enhancements
- The technical specifications promote standardization by ensuring that data collected by PSOs and other entities are clinically and electronically comparable. The specifications also provide direction to software developers, so the Common Formats can be implemented electronically, and to PSOs, so the Common Formats can be submitted electronically to the PSO Privacy Protectio…
Menting on Common Formats Version 1.1
- To allow for greater participation by the private sector in the subsequent development of the Common Formats, AHRQ engaged the National Quality Forum (NQF), a non-profit organization focused on healthcare quality, to solicit comments and advice to guide the further refinement of the Common Formats. The NQF began this process with feedback on AHRQ's 0.1 Beta release o…
Popular Posts:
- 1. coughlan patient portal
- 2. dch tuscaloosa patient portal
- 3. patient portal engagement tool
- 4. collom and carney patient portal
- 5. maam exams patient portal
- 6. hhsi patient portal
- 7. gmh patient portal
- 8. patient portal physicians and midwives
- 9. brookdale hospital patient portal
- 10. patient portal marquette mi