Thalassemia - StatPearls - NCBI Bookshelf
10 hours ago · Several laboratory tests have been developed to screen and diagnose thalassemia: Complete blood count (CBC): CBC is often the first investigation in a suspected case of thalassemia. A CBC showing low hemoglobin and low MCV is the first indication of thalassemia, after ruling out iron deficiency as the cause of anemia. >> Go To The Portal
The initial workup for a patient with suspected thalassemia should include a complete blood count (CBC), review of the blood smear, and iron studies, as follows: CBC - Thalassemia involves microcytic anemia with a high red blood cell count, with the high count possibly helping to distinguish between thalassemia and iron deficiency [ 1]
Full Answer
What does a CBC look like with thalassemia?
If any person has thalassemia, his/her red blood cells appear small in size as compared to the normal ones i.e., low MCV. Even red cells may- Hypochromic i.e. pale as compared to normal ones
What blood tests are done to detect thalassemia?
- A low count of MCV usually can be used in detecting thalassemia
- If MCV is low and iron deficiency is not a cause of that MCV, then thalassemia is detected
- Blood smear test is also done, which is performed by taking a thin layer of blood and treating it with a special stain
How is thalassemia diagnosed?
- Blood transfusions from healthy donors, which can alleviate anemia. ...
- Iron chelation therapy, which reduces the amount of iron in the body and prevents or treats iron overload.
- Stem cell transplantation to replace the blood-forming stem cells with the defective hemoglobin gene (s). ...
How to treat thalassemia?
They say they have spent all their income and savings on the treatment of their daughter and are now financially stretched thin. Diksha Raule, aged eight, of Babai Rural Municipality-6 in Dang district has also been suffering from beta thalassemia for the ...

Does thalassemia show on CBC?
Doctors diagnose thalassemias using blood tests, including a complete blood count (CBC) and special hemoglobin tests. A CBC measures the amount of hemoglobin and the different kinds of blood cells, such as red blood cells, in a sample of blood.
What does a CBC look like with thalassemia?
A complete blood count (CBC), which includes measures of hemoglobin and the quantity (and size) of red blood cells. People with thalassemias have fewer healthy red blood cells and less hemoglobin than normal; those with alpha or beta thalassemia trait may have smaller-than-normal red blood cells.
What are the lab values for thalassemia?
The most widely used cutoff values of MCV and MCH for indicating thalassemia are 79 fl and 27 pg, respectively 14. Reticulocytes are normal or slightly increased, but they do not have diagnostic value.
What is normal report of thalassemia?
Thalassemia major is characterized by reduced Hb level (<7 g/dl), mean corpuscolar volume (MCV) > 50 < 70 fl and mean corpuscolar Hb (MCH) > 12< 20 pg. Thalassemia intermedia is characterized by Hb level between 7 and 10 g/dl, MCV between 50 and 80 fl and MCH between 16 and 24 pg.
Why is RBC count high in thalassemia?
In beta-thalassemia major, anemia is severe, often with hemoglobin ≤ 6 g/dL (≤ 60 g/L). Red blood cell count is elevated relative to hemoglobin because the cells are very microcytic.
Does low MCV mean thalassemia?
Patients who have thalassemia have an anemia associated with microcytosis (low MCV) and hypochromia (low MCH), although the extent of anemia can be highly variable.
Is Rdw high in thalassemia?
Until now, many studies have shown a high efficacy of RDW in distinguishing IDA from the thalassemia trait, but this has not been the same for thalassemia disease. Our study used RDW for differentiating between IDA and NTDT in adults with moderate to severe microcytic anemia, according to the WHO classification.
Can normal blood test detect thalassemia?
If your doctor suspects your child has thalassemia, he or she can confirm a diagnosis with blood tests. Blood tests can reveal the number of red blood cells and abnormalities in size, shape or color. Blood tests can also be used for DNA analysis to look for mutated genes.
How can you differentiate between iron-deficiency anemia and thalassemia depending on CBC?
The two best measures or calculations from the CBC are the Red Blood Cell count alone (RBC) and the Mentzer Index (MCV/RBC). An RBC above 5 x 1012/l is often seen in thalassemia, while a count <5 is more typical of iron deficiency.
Is hemoglobin low in thalassemia?
Thalassemia (thal-uh-SEE-me-uh) is an inherited blood disorder that causes your body to have less hemoglobin than normal. Hemoglobin enables red blood cells to carry oxygen. Thalassemia can cause anemia, leaving you fatigued. If you have mild thalassemia, you might not need treatment.
What is the normal level of HbA2 in blood?
Normal Results In adults, these are normal percentages of different hemoglobin molecules: HbA: 95% to 98% (0.95 to 0.98) HbA2: 2% to 3% (0.02 to 0.03)
Why RDW is normal in thalassemia?
The RDW is normal in patients with thalassemia and anemia of chronic disease but high in those with iron deficiency. The MCV is decreased in iron-deficiency anemia and in thalassemia minor and normal or decreased in chronic disease.
What is a thalassemia clinical research network?
The Thalassemia Clinical Research Network (TCRN) was a multi-institutional, NIH-sponsored network established to evaluate clinical complications and treatment strategies for patients with thalassemia. Several studies have emerged from this network and have been fully described elsewhere (Table I). The Thalassemia Longitudinal Cohort Study (TLC), launched in 2007, was a longitudinal registry designed to measure the prevalence and incidence of complications of thalassemia. In the design of the TLC, a consensus among TCRN investigators for routine monitoring of clinically relevant measures was developed (Table II). This established a data set collected from over 400 North American and British patients [3]. In this paper, we describe the measures adopted by the TLC investigators and review available literature to provide guidelines for monitoring and management of patients with thalassemia. We describe guidelines for monitoring of iron overload and pain, as well as for transfusion and stem cell transplantation. We also briefly review important findings regarding quality of life. A full list of TCRN publications is included in Supplement A.
What percentage of patients with thalassemia have low bone density?
Approximately 60-75% of adult patients with thalassemia have reduced bone mass for age, defined as a bone mineral density Z-score <−2.0, regardless of transfusion status [34]. In the TCRN, the proportion of patients with low bone mineral density increased with age. Low bone mass is found in 8.7% of patients under 10 years of age, 44% in the 11-19 year-old group, and in 61% of patients over 20. Patients with thalassemia have many risk factors that adversely affect bone mass including: bone marrow hyperplasia, anemia, poor growth, endocrinopathies, decreased physical activity and low vitamin D levels [34].
What is a thalassemia registry?
A registry to characterize demographic and clinical features of patients with thalassemia
What is a RBC transfusion?
Red blood cell (RBC) transfusions are the principal supportive intervention for patients with thalassemia major (TM), and are used intermittently in thalassemia intermedia. In patients with TM, transfusion therapy is often initiated before one year of age [4–6]. Complications directly related to transfusion include blood-borne infections, development of anti-RBC antibodies (both auto- and alloimmunization), and allergic, febrile or delayed hemolytic transfusion reactions.
What causes growth failure in thalassemia?
Growth failure occurs in 25-28% of patients, regardless of the thalassemia syndrome [25]. Contributing factors include chronic anemia or inadequate transfusion support, chelation toxicity, nutritional deficiencies, growth hormone deficiency, and other iron-as sociated endocrinopathies as outlined above [25]. Historically, thalassemia has been associated with marked osseous changes, including frontal bossing, maxillary hyperplasia, and limb deformities, which are attributed to bone marrow hyperplasia and cortical thinning due to massive ineffective erythropoiesis. The introduction of regular transfusions in the mid 1960's enabled patients to maintain a near-normal hemoglobin level resulting in improvement or prevention of bone deformities. However, since transfusion and chelation therapies were introduced, growth patterns in thalassemia have not significantly improved [34].
What is the prevalence of hypothyroidism in thalassemia?
The prevalence of hypothyroidism in thalassemia is approximately 8-10%, thus annual screening with free thyroxine (T4) and TSH concentration is recommended [2, 25]. Hypoparathyroidism occurs in 2% of North American patients with thalassemia and is associated with severe iron overload [25, 33].
What are the most common inherited diseases?
Hemoglobinopathies, including sickle cell disease and thalassemia, are among the most common inherited disorders worldwide [1]. Thalassemia represents a group of disorders resulting from impaired hemoglobin synthesis and ineffective erythropoiesis. For patients with more severe forms of thalassemia, chronic lifelong blood transfusions are the mainstay of therapy. Untransfused children with severe thalassemia often do not survive beyond age 5 years. With transfusions and comprehensive care, birth cohorts followed from 1970 have shown life expectancy extending into the 4thdecade of life and beyond [2]. Routine transfusions have been life sustaining; however, complications of chronic blood exposure are now the predominant challenges in disease management.
What is CBC in blood work?
A complete blood count (CBC), which includes measures of hemoglobin and the quantity (and size) of red blood cells. People with thalassemias have fewer healthy red blood cells and less hemoglobin than normal; those with alpha or beta thalassemia trait may have smaller-than-normal red blood cells.
How many proteins are in hemoglobin?
Four protein chains make up hemoglobin — 2 alpha globin and 2 beta globin chains. There are 2 major types of thalassemia – alpha thalassemia and beta thalassemia – named after defects that can occur in these protein chains.
How many beta globin genes are there?
There are normally 2 beta globin genes, one from each parent. Beta thalassemia is a change in 1 or both of the beta globin genes. This chart describes the different types of beta thalassemia.
Why do we need iron chelation?
Iron chelation is removal of excess iron from the body. A danger with blood transfusions is that they can cause iron overload, which may in turn causedamage to other organs.
What are the symptoms of beta thalassemia?
Symptoms of beta thalassemia include growth problems, bone abnormalities such as osteoporosis, and an enlarged spleen (the organ in the abdomen that plays a part in fighting infection). People with thalassemia can get too much iron in their bodies (iron overload), either from frequent blood transfusions or from the disease itself.
Why do people with thalassemia have infections?
People who have thalassemias may suffer severe infections. One reason may be the large number of blood transfusions these patients need; the infections may be carried in the blood that they receive in a transfusion.
What is the treatment for thalassemia?
Standard treatments for patients with thalassemia major are blood transfusions and iron chelation. Blood transfusion involves injection of red blood cells through a vein to restore normal levels of healthy red blood cells and hemoglobin.
What happens when a mutation in a gene causes a hemolytic anemia?
Mutations to both β genes results in severely decreased or absent production of β globin chains. Excess α globin chains are unable to form tetramers leading to their precipitation and accumulation in the red blood cell. This damages the cell and results in a chronic and severe hemolytic anemia.
How much hemoglobin is present at birth?
There is between 5-15% hemoglobin Barts present at birth, but this decreases once β globin chain production takes over and γ globin chain production decreases. In adults, globin chain production is balances, so no Hemoglobin H is formed.
Why is HB Barts incompatible with life?
Because no sustainable amount of α globin chains is produced, this state is usually considered to be incompatible with life. Excess γ globin chains result in the formation of Hb Barts. Due to its high affinity for oxygen, it is not able to efficiently transport oxygen to the tissues of the developing fetus.
What happens when three genes are deleted?
Patients present with a chronic hemolytic anemia that varies from mild to moderate. Patients are transfusion- independent.
What blood smears show thalassemia?
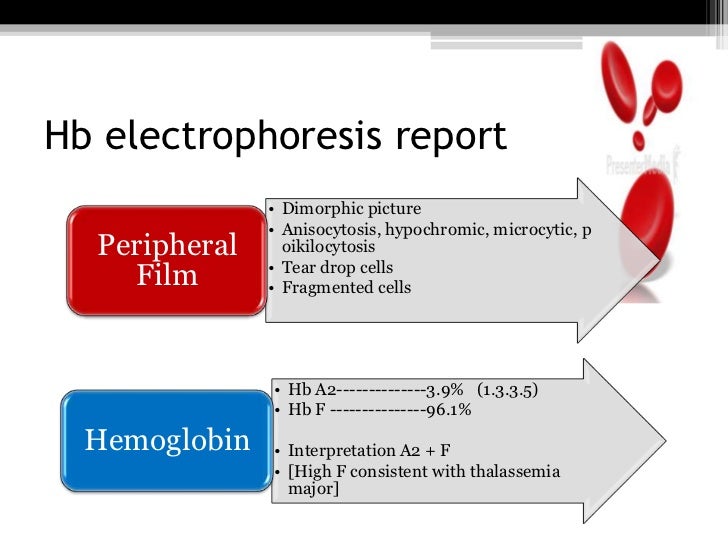
Images show thalassemia peripheral blood smears with hypochromic, microcytic red blood cells and poikilocytosis. From MLS Collection, University of Alberta.
Where is the globin chain located?
Cause (s): α globin chain genes are located on chromosome 16 and there are normally four genes in total (αα/αα), two inherited from each parent. α-thalassemia results when there is a deletion in any number of the α globin gene.
Is a benign HB Barts mutation asymptomatic?
Patient is asymptomatic and the mutation is benign. In newborns, there is an excess production of γ globin chains. These γ globin chains tend to also form tetramers and result in Hemoglobin Barts (Hb Barts). Hb Barts has a high oxygen affinity and is inefficient for oxygen delivery to the tissues of the developing fetus.
How do I know if I have thalassemia?
People with moderate and severe forms of thalassemia usually find out about their condition in childhood, since they have symptoms of severe anemia early in life. People with less severe forms of thalassemia may only find out because they are having symptoms of anemia, or maybe because a doctor finds anemia on a routine blood test or a test done for another reason.
What is the name of the part of the body that carries oxygen to all cells?
When thalassemia is called “alpha” or “beta,” this refers to the part of hemoglobin that isn’t being made.
How are thalassemia traits passed down?
In the same way that traits for hair color and body structure are passed down from parents to children, thalassemia traits are passed from parents to children. The type of thalassemia that a person has depends on how many and what type of traits for thalassemia a person has inherited, or received from their parents.
What does "major" mean in a trait?
When the words “trait,” “minor,” “intermedia,” or “major” are used, these words describe how severe the thalassemia is. A person who has thalassemia trait may not have any symptoms at all or may have only mild anemia, while a person with thalassemia major may have severe symptoms and may need regular blood transfusions.
What is a blood disorder that is caused when the body doesn't make enough of a protein called hemoglob?
minus. Related Pages. Thalassemia is an inherited (i.e., passed from parents to children through genes) blood disorder caused when the body doesn’t make enough of a protein called hemoglobin, an important part of red blood cells.
Why are there fewer red blood cells in the bloodstream?
When there isn’t enough hemoglobin, the body’s red blood cells don’t function properly and they last shorter periods of time , so there are fewer healthy red blood cells traveling in the bloodstream. Red blood cells carry oxygen to all the cells of the body. Oxygen is a sort of food that cells use to function.
What are the two types of thalassemia?
When we talk about different “types” of thalassemia, we might be talking about one of two things: the specific part of hemoglobin that is affected (usually either “alpha” or “beta”), or the severity of thalassemia, which is noted by words like trait, carrier, intermedia, or major. Hemoglobin, which carries oxygen to all cells in the body, ...
How is thalassemia treated?
Hear Robert’s tips on how to successfully transition to adult care for thalassemia.
How does iron build up in the body?
Red blood cells contain a lot of iron, and over time, the iron from all of the transfusions can build up in the body. When it builds up, the iron collects in places like the heart, liver, and brain, and can make it hard for these organs to work properly. To prevent iron overload, people with thalassemia may need chelation therapy, which is when doctors give a medicine – either a pill or a shot under the skin – to remove excess iron before it builds up in the organs.
Why is thalassemia considered immunocompromised?
Because of this, people with thalassemia are said to be “immunocompromised,” which means that some of the body’s defenses against infection aren’t working. When you are immunocompromised, it is easier for you to get infections and you sometimes need extra protection, like flu shots and other vaccines.
What is the best vitamin for thalassemia?
Many times people with thalassemia are prescribed a supplemental B vitamin, known as folic acid, to help treat anemia. Folic acid can help red blood cells develop. Treatment with folic acid is usually done in addition to other therapies.
How to tell if you have thalassemia?
Since your body has fewer red blood cells when you have thalassemia, you may have symptoms of a low blood count, or anemia. When you have anemia, you might feel tired or weak. You might also experience: 1 Dizziness 2 Shortness of breath 3 A fast heart beat 4 Headache 5 Leg cramps 6 Difficulty concentrating 7 Pale skin
Do people with thalassemia need blood transfusions?
People with thalassemia minor or trait usually do not need blood transfusions because they either do not have anemia or have only a mild anemia.
Where is the spleen located?
It sits on the left side of your abdomen, just under your lower ribs. The spleen has many other jobs. Two of the major ones are filtering the blood and monitoring the blood for certain infections. When it finds these infections, it can start the process of fighting them.
What is the best treatment for thalassemia in children?
Stem cell transplant. Also called a bone marrow transplant, a stem cell transplant might be an option in some cases. For children with severe thalassemia, it can eliminate the need for lifelong blood transfusions and drugs to control iron overload.
What is the best supplement for bone health?
Your doctor might also recommend a folic acid supplement to help your body make new red blood cells. To keep your bones healthy, make sure your diet contains enough calcium and vitamin D. Ask your doctor what the right amounts are for you and whether you need a supplement.
How to manage thalassemia?
You can help manage your thalassemia by following your treatment plan and adopting healthy-living habits. Avoid excess iron. Unless your doctor recommends it, don't take vitamins or other supplements that contain iron. Eat a healthy diet. Healthy eating can help you feel better and boost your energy.
How to tell if a child has thalassemia?
If your doctor suspects your child has thalassemia, he or she can confirm a diagnosis with blood tests. Blood tests can reveal the number of red blood cells and abnormalities in size, shape or color.
How to avoid spleen infection?
Avoid infections. Wash your hands frequently and avoid sick people . This is especially important if you've had your spleen removed.
Can thalassemia cause excess iron?
Some people with thalassemia who don't have regular transfusions can also develop excess iron. Removing the excess iron is vital for your health. To help rid your body of the extra iron, you might need to take an oral medication, such as deferasirox (Exjade, Jadenu) or deferiprone (Ferriprox).
When should a baby be tested for thalassemia?
Testing can be done before a baby is born to find out if he or she has thalassemia and determine how severe it might be. Tests used to diagnose thalassemia in fetuses include:
Are you sure your patient has thalassemia? What should you expect to find?
Patients who have thalassemia have an anemia associated with microcytosis (low MCV) and hypochromia (low MCH), although the extent of anemia can be highly variable.
What laboratory studies should you order to help make the diagnosis and how should you interpret the results?
A complete blood count is helpful initially to demonstrate the extent of anemia and to also show that the patient has hypochromia and microcytosis.
What other clinical manifestations may help me to diagnose thalassemia?
Many patients with thalassemia will have hepatosplenomegaly as a result of extramedullary hematopoiesis, which should be assessed on clinical exam. Furthermore, signs of medullary expansion such as frontal bossing can be helpful in assessing the severity of thalassemia.
What other additional laboratory studies may be ordered?
As noted above, genetic testing to delineate specific mutations may be useful for predicting the clinical course in certain patients. However, limitations exist to make genotype and phenotype correlations. Recent studies suggest that other genetic variation may contribute to the variation in clinical phenotype and therefore in the future, such testing may be of benefit to patients with thalassemia.
What happens to the remaining globin chains?
The remaining globin chains precipitate in erythroid precursors and in red blood cells, resulting in an anemia from either ineffective production of red blood cells, hemolysis of red blood cells, or a combination of these effects. In the case of β-thalassemia, which is due to defective production of the β-globin chain of hemoglobin, ...
Why is thalassemia common?
The thalassemias are among the most common genetic diseases worldwide and are attributable to unbalanced production of the hemoglobin molecule, due to either insufficient production of the α- or β-globin chains . The remaining globin chains precipitate in erythroid precursors and in red blood cells, resulting in an anemia from ...
What happens to hemoglobin in thalassemia?
In α-thalassemia, reduced production of α-globin results in tetramers of β-globin known as hemoglobin H (HbH) that can precipitate within mature red blood cells. This in turn results in an anemia from hemolysis and destruction of these red blood cells, as well as some ineffective production of such red blood cells.