Guideline on writing a case report - PMC
3 hours ago · This learning experience, based on the observation of clinical cases, can be transferred to others, locally, nationally, and internationally, through communication and reporting. ... The source of case reports is clinical setting, every single patient is a potential case report therefore, always keep an eye on unusual cases in your practice ... >> Go To The Portal
Testimony and evidence presented at trial An 84-year-old patient was transferred from a hospital to the nursing home. She had osteoarthritis and osteoporosis, which required her to be bed bound and dependent on nursing staff and others in the home to transfer her in and out of bed.
Full Answer
How do you write a case report for a patient?
Develop a descriptive and succinct working title that describes the phenomenon of greatest interest (symptom, diagnostic test, diagnosis, intervention, outcome). WHAT happened. Gather the clinical information associated with patient visits in this this case report to create a timeline as a figure or table.
What is a case report in medical terms?
A case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine.
Are published case reports useful for optimal patient care?
Published case reports provide essential information for optimal patient care because they can describe important scientific observations that are missed or undetected in clinical trials, and provide individual clinical insights thus expanding our knowledge base [ 3 ].
Why publish biomedical case reports?
Many biomedical journals publish case reports and provide authors with guidelines that provide instruction for acceptance criteria, content, and format and give advice on relevant patient case reports that merit publication [ 3 ].
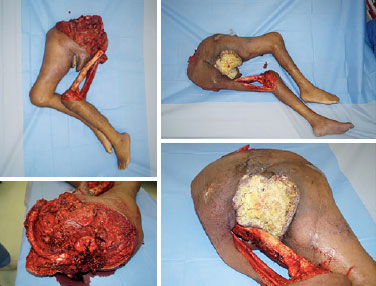
Do case reports count as publications for residency?
However, don't forget that poster presentations, oral presentations, online articles, and/or case reports also count as research publications in ERAS that you can complete by the time you apply for residency.
How do you write a case report to a patient?
III. Patient case presentationDescribe the case in a narrative form.Provide patient demographics (age, sex, height, weight, race, occupation).Avoid patient identifiers (date of birth, initials).Describe the patient's complaint.List the patient's present illness.List the patient's medical history.More items...•
Do you need patient permission for case report?
Don't publish a case report without the patient's consent As explained above, informed patient consent is mandatory for the publication of your case reports. Ignoring this requirement can result in a rejection for your work and worse, ruin your relationship and reputation with patients.
What does a case report include?
A case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine.
What is the purpose of the case report form?
A Case Report Form (CRF) is a printed or electronic document that is created and used in clinical trial research to capture standardised clinical data from each patient separately and to transfer it to Data Management.
What is a patient case presentation?
A patient case presentation is a demonstration of a learner's knowledge and skills related to the management of disease states and drug therapies through application to an actual patient case. Typical Information Included in a Patient Case Presentation. 1.
Where can I publish my case report?
Where to Publish Case ReportsElsevier Journal Finder.Edanz Journal Selector.EndNote Manuscript Matcher.Springer.
What is the importance of case reports?
Case reports are valuable resources of unusual information that may lead to new research and advances in clinical practice. Many journals and medical databases recognize the time-honored importance of case reports as a valuable source of new ideas and information in clinical medicine.
What does a case report look like?
Case reports should encompass the following five sections: an abstract, an introduction with a literature review, a description of the case report, a discussion that includes a detailed explanation of the literature review, and a brief summary of the case and a conclusion.
What type of evidence is a case report?
Case reports are considered the lowest level of evidence, but they are also the first line of evidence, because they are where new issues and ideas emerge. This is why they form the base of our pyramid. A good case report will be clear about the importance of the observation being reported.
What to ask for in a case report?
It is often best to ask for informed consent and the patient’s perspective before you begin writing your case report. Appendices (If indicated). Submission to a scientific journal. Follow author guidelines and journal submission requirements when writing and submitting your case report to a scientific journal.
Do you need informed consent for a journal?
The patient should provide informed consent (including a patient perspective) and the author should provide this information if requested. Some journals have consent forms which must be used regardless of informed consents you have obtained. Rarely, additional approval (e.g., IRB or ethics commission) may be needed.
What is a case report?
Case Report: A Beginner’s Guide with Examples. A case report is a descriptive study that documents an unusual clinical phenomenon in a single patient. It describes in details the patient’s history, signs, symptoms, test results, diagnosis, prognosis and treatment.
Why do we need case reports?
Case reports offer, in general a fast, easy and cheap way to report an unusual observation or a rare event in a clinical setting, as these have very small probability of being detected in an experimental study because of limitations on the number of patients that can be included.
What is an example of recovery from the passage of an iron bar through the head?
This is an interesting case of a construction foreman named Phineas Gage. [ Source] In 1848, due to an explosion at work, an iron bar passed through his head destroying a large portion of his brain’s frontal lobe.
Is a case report causal?
Observing a relationship between an exposure and a disease in a case report does not mean that it is causal in nature. The absence of a control group that provides a benchmark or a point of reference against which we compare our results. A control group is important to eliminate the role of external factors which can interfere with ...
Is a case report representative of the entire population?
So, results from a case report cannot be representative of the entire population.
What should I do after writing a case report?
Once you have written a draft of the case report, you should seek feedback on your writing, from experts in the field if possible, or from those who have written case reports before .
What is required to submit a case report?
Journals often have specific requirements for publishing case reports, which could include a requirement for informed consent, a letter or statement from the IRB and other things.
Why is it important to write a case report?
the reason you would go to the trouble of writing one, is that the case is sufficiently unique, rare or interesting such that other medical professionals will learn something from it.
Does CHM have funding for publication?
It is best practice to check the journal's Info for Authors section or Author Center to determine what the cost is to publish. CHM does NOT have funds to support publication costs, so this is an important step if you do not want to pay out of pocket for publishing.
Is it free to publish a case report?
Be aware that it may not be free to publish your case report. Many journals charge publication fees. Of note, many open access journals charge author fees of thousands of dollars. Other journals have smaller page charges (i.e. $60 per page), and still others will publish for free, with an "open access option".
Do you need informed consent for a case report?
Although not technically required, especially if the case report does not include any identifying information, some journals require informed consent for all case reports. The CARE guidelines recommend obtaining informed consent AND the patient's perspective on the treatment/outcome (if possible).
Do journals have informed consent?
Journals may have their own informed consent form that they would like you to use, so please look for this when selecting a journal. Once you've identified the case, selected an appropriate journal (s), and considered informed consent, you can collect the required information to write the case report.
What is a case report?
A case report is an unsystematic clinical observation that states the outcome or response of a single patient to a diagnostic strategy or treatment . Case reports serve to document and share novel cases amongst the medical community for educational purposes.
When case reports describe or discuss unique or rare circumstances, as they often do, it may be difficult or impossible to answer
When case reports describe or discuss unique or rare circumstances, as they often do, it may be difficult or impossible to de-identify those cases such that there is no reasonable expectation that the individuals included can be identified, so patient authorization generally would be required.
What is PHI in healthcare?
This is known as safe harbor de-identification.
When safe harbor de-identification is not possible or the opportunity to identify the patient exists, even after de-ident
When safe harbor de-identification is not possible or the opportunity to identify the patient exists, even after de-identification, the expert determination method for de-identification can be considered. For purposes of de-identification, an expert is defined as: A person with appropriate knowledge of and experience with generally accepted statistical and scientific principles and methods for rendering information not individually identifiable:
Is PHI de-identified under HIPAA?
It is important to understand that determining whether data are de-identified under HIPAA is a more restrictive determination than determining whether private information is individually identifiable under the Common Rule. The HIPAA rule considers PHI as any information that may identify an individual; was created or received by a member of a HIPAA covered entity; and relates to the individual's past, present, or future physical/mental health or condition, health care, or payment for health care. HIPAA recognizes two methods for de-identification of data.
What is a case report?
Wikipedia [ 9] has this to say: “In medicine, a case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports may contain a demographic profile of the patient, but usually describe an unusual or novel occurrence.
What is the introduction of a case report?
The Introduction ( JMCR) or Background ( BMCRN) section should explain the background of the case, including the disorder, usual presentation and progression, and an explanation of the presentation if it is a new disease. If it is a case discussing an adverse drug interaction the Introduction should give details of the drug’s common use and any previously reported side effects. It should also include a brief literature review. This should give an introduction to the case report from the standpoint of those without specialist knowledge in the area, clearly explaining the background of the topic. It should end with a very brief statement of what is being reported in the article.
What is the author's information section?
This section includes any relevant information about the author (s) that may aid the reader’s interpretation of the article and understanding of the standpoint of the author (s ). This may include details about the authors’ qualifications, current positions they hold at institutions or societies, or any other relevant background information. Please refer to authors using their initials. Note this section should not be used to describe any competing interests.
What should be included in a case presentation?
The Case presentation section should contain a description of the patient’s relevant demographic information (with out adding any details that could lead to the identification of the patient); any relevant medical history of the patient; the patient's symptoms and signs; any tests that were carried out and a description of any treatment or intervention. If it is a case series, then details must be included for all patients. This section may be broken into subsections with appropriate subheadings.
Why are case reports important?
Published case reports provide essential information for optimal patient care because they can describe important scientific observations that are missed or undetected in clinical trials, and provide individual clinical insights thus expanding our knowledge base [ 3 ].
When did BioMed Central start publishing case reports?
The Case Report section of BioMed Central Research Notes was created and began publishing case reports in 2012. Between the two of them, thousands of peer-reviewed case reports have now been published with a worldwide audience.
Do you acknowledge the patient on whom the case report is based?
Authors may also like to acknowledge ( anonymously) the patient on whom the case report is based. If a scientific (medical) writer is used, this person should be included in the Acknowledgements section, including their source (s) of funding.

Advantages of Case Reports
Limitations of Case Reports
- Part 1 — Working Title, WHAThappened: Timeline and Narrative
1. Develop a descriptive and succinct working title that describes the phenomenon of greatest interest (symptom, diagnostic test, diagnosis, intervention, outcome). 2. WHAT happened. Gather the clinical information associated with patient visits in this this case report to create a timeline… - Part 2 — WHYit might have happened: Introduction, Discussion, Conclusion
1. The introduction should briefly summarize why this case report is important and cite the most recent CARE article (Riley DS, Barber MS, Kienle GS, AronsonJK, et al. CARE guidelines for case reports: explanation and elaboration document. JClinEpi 2017 Sep;89:218-235. doi: 10.1016/jclin…
Real-World Examples of Case Reports
References
- Case reports offer, in general a fast, easy and cheap way to report an unusual observation or a rare event in a clinical setting, as these have very small probability of being detected in an experimental study because of limitations on the number of patients that can be included. These events deserve to be reported since they might provide insights on some exceptions to general r…
Further Reading
- Observing a relationship between an exposure and a disease in a case report does not mean that it is causal in nature. This is because of: 1. The absence of a control group that provides a benchmark or a point of reference against which we compare our results. A control group is important to eliminate the role of external factors which can interfere with the relationship betw…
Popular Posts:
- 1. mayo clinic patient portal logging me out every time i click on something
- 2. lgmc patient portal login
- 3. torrance patient portal
- 4. umass memorial medical center find a patient portal
- 5. el paso county patient portal
- 6. patient portal village square internal medicine
- 7. sonoma valley hospital patient portal
- 8. st joseph health exchange patient portal team
- 9. patient portal for self started medical business
- 10. hamilton orthopedics patient portal