Informed Consent for Case Reports - PMC
10 hours ago Informed consent—or, as it is sometimes written, truly informed consent—may be impossible for psychotherapy patients because of transference. 21 In our attempt to be legally and ethically correct, we have to ignore what has been known for almost a full century about patients' hidden attitudes toward therapists and physicians. 22 Mental health professionals ought to be able to recognize that obtaining truly informed consent for publication of a … >> Go To The Portal
The CARE guidelines recommend obtaining informed consent from patients for all case reports. Our recommendation is to obtain informed consent from the patient. Although not technically required, especially if the case report does not include any identifying information, some journals require informed consent for all case reports before publishing.
Full Answer
Do patients read consent forms?
They often do not read consent forms carefully because they assume that someone else has scrutinised the risks and benefits on their behalf. Interviews with 103 patients showed that many factors influence a decision to take part in medical research (Hastings Center Report 1996;26 (5):25-9).
Is your medical consent properly informed?
Informed consent to medical treatment is fundamental in both ethics and law. Patients have the right to receive information and ask questions about recommended treatments so that they can make well-considered decisions about care. Successful communication in the patient-physician relationship fosters trust and supports shared decision making.
What is a patient consent?
The physician provided his patient with a dense seven-page consent form, in which specific surgical risks, like tissue necrosis, were mentioned; she signed the consent form. However, the physician did not engage in any detailed discussions with his patient ...
Is informed consent possible?
Informed consent is not only required for clinical trials but is an essential prerequisite before enrolling each and every participant in any type of research involving human subjects including; diagnostic, therapeutic, interventional, bioequivalence, social and behavioral studies and for all research conducted domestically or abroad.
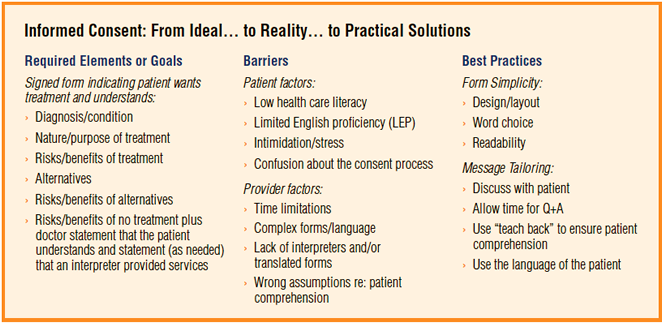
Do you need a consent from patient for case report?
Although not technically required, especially if the case report does not include any identifying information, some journals require informed consent for all case reports before publishing. The CARE guidelines recommend obtaining informed consent AND the patient's perspective on the treatment/outcome (if possible).
How do you consent a patient to a case report?
For a patient's consent to publication of information about them in a journal or thesisThe Information will be published without my name/child's name/relatives name attached and every attempt will be made to ensure anonymity. ... The Information may be published in a journal which is read worldwide or an online journal.More items...
Do case reports require ethical approval?
Case reports and studies intended for quality improvement are often considered not research and do not need IRB approval. Nevertheless, there should be some processes of clearing those studies with respect to ethical handling of patients and related data.
Do you need IRB approval for case report?
Under HIPAA, a single case report is an activity to develop information to be shared for medical/educational purposes. Therefore, the use of protected health information to prepare a paper for publication of a single case report does not require IRB review for HIPAA purposes.
What is a patient consent form?
The main purpose of the informed consent process is to protect the patient. A consent form is a legal document that ensures an ongoing communication process between you and your health care provider.
What types of consent are there?
What are the Different Types of Consent?Informed consent.Implied consent.Explicit consent.Active consent.Passive consent.Opt-Out consent.Key takeaway.
How do you write ethics approval and consent to participate?
Ethics approval and consent to participate include a statement on ethics approval and consent (even where the need for approval was waived) include the name of the ethics committee that approved the study and the committee's reference number if appropriate.
What is ethical approval?
1. A component of the research process, whose purpose is to protect both the researcher and the participants in the research, which should have their dignity, rights, safety, and welfare respected.
Do you need informed consent for retrospective studies?
Consent is an ethical issue. Usually it is obtained from participant persons, their guardian or sponsors, regardless of whether the study is retrospective (e.g., retrospective interview) or prospective. However, if the retrospective study is based on recorded data, then approval from owners of the data is required.
Does case report count as research?
The case report is a research design where an unexpected or novel occurrence is described in a detailed report of findings, clinical course, and prognosis of an individual patient, which might be, but not mandatory, accompanied by a review of the literature of other reported cases.
Are case studies exempt from IRB review?
As a result, case studies generally qualify for exempt review by the IRB provided that the study (a) does not involve a sensitive topic, (b) is conducted in a manner that protects subjects' identity, and (c) does not involve at-risk or special populations.
What is the difference between case study and case report?
Case studies are widely used in psychology to provide insight into unusual conditions. A case study, also known as a case report, is an in depth or intensive study of a single individual or specific group, while a case series is a grouping of similar case studies / case reports together.
What is required to submit a case report?
Journals often have specific requirements for publishing case reports, which could include a requirement for informed consent, a letter or statement from the IRB and other things.
What should I do after writing a case report?
Once you have written a draft of the case report, you should seek feedback on your writing, from experts in the field if possible, or from those who have written case reports before .
Why is it important to write a case report?
the reason you would go to the trouble of writing one, is that the case is sufficiently unique, rare or interesting such that other medical professionals will learn something from it.
Does CHM have funding for publication?
It is best practice to check the journal's Info for Authors section or Author Center to determine what the cost is to publish. CHM does NOT have funds to support publication costs, so this is an important step if you do not want to pay out of pocket for publishing.
Is it free to publish a case report?
Be aware that it may not be free to publish your case report. Many journals charge publication fees. Of note, many open access journals charge author fees of thousands of dollars. Other journals have smaller page charges (i.e. $60 per page), and still others will publish for free, with an "open access option".
Do you need informed consent for a case report?
Although not technically required, especially if the case report does not include any identifying information, some journals require informed consent for all case reports. The CARE guidelines recommend obtaining informed consent AND the patient's perspective on the treatment/outcome (if possible).
Do journals have informed consent?
Journals may have their own informed consent form that they would like you to use, so please look for this when selecting a journal. Once you've identified the case, selected an appropriate journal (s), and considered informed consent, you can collect the required information to write the case report.
Does a doctor have to disclose your name?
Dr. (insert name) is obligated to protect your privacy and not disclose your personal information (information about you and your health that identifies you as an individual e.g. name, date of birth, medical record number). When the case report is published or presented, your identity will not be disclosed.
Can a case report be published?
A case report may be published ( in print and/or via internet dissemination) for others to read, and/or presented at a conference. This form explains the purpose of this case report. Please read this form carefully and take your time to make your decision and ask any questions that you may have.
What to ask for in a case report?
It is often best to ask for informed consent and the patient’s perspective before you begin writing your case report. Appendices (If indicated). Submission to a scientific journal. Follow author guidelines and journal submission requirements when writing and submitting your case report to a scientific journal.
Do you need informed consent for a journal?
The patient should provide informed consent (including a patient perspective) and the author should provide this information if requested. Some journals have consent forms which must be used regardless of informed consents you have obtained. Rarely, additional approval (e.g., IRB or ethics commission) may be needed.
How many authors should be in a case report?
Case reports should have a maximum of four authors, of which at least one must have been involved in the patient’s care. All authors must have made an individual contribution to the writing of the article and not just been involved with the patient’s care.
Is BMJ a copyright?
Copyright and authors’ rights. BMJ Case Reports authors are required to grant B MJ an assignment of the copyright in the report unless an author is a Crown employees or where BMJ has agreed CC BY applies, in which case a non exclusive licence is granted to BMJ.
Does BMJ accept case series?
BMJ Case Reports does not accept case series. However, if we feel that an article makes a point better by including more than one case, we will consider the article. If your case report involves more than three patients, please contact the editorial office so that we can assess your case. Each case will be peer reviewed by at least two external ...
Is BMJ a preprint?
BMJ does not consider the posting of an article in a dedicated preprint repository to be prior publication. Preprints are reports of work that have not been peer-reviewed; Preprints should therefore not be used to guide clinical practice, health-related behaviour or health policy.
What is a case report?
A case report is an unsystematic clinical observation that states the outcome or response of a single patient to a diagnostic strategy or treatment . Case reports serve to document and share novel cases amongst the medical community for educational purposes.
When case reports describe or discuss unique or rare circumstances, as they often do, it may be difficult or impossible to answer
When case reports describe or discuss unique or rare circumstances, as they often do, it may be difficult or impossible to de-identify those cases such that there is no reasonable expectation that the individuals included can be identified, so patient authorization generally would be required.
When safe harbor de-identification is not possible or the opportunity to identify the patient exists, even after de-ident
When safe harbor de-identification is not possible or the opportunity to identify the patient exists, even after de-identification, the expert determination method for de-identification can be considered. For purposes of de-identification, an expert is defined as: A person with appropriate knowledge of and experience with generally accepted statistical and scientific principles and methods for rendering information not individually identifiable:
Is PHI de-identified under HIPAA?
It is important to understand that determining whether data are de-identified under HIPAA is a more restrictive determination than determining whether private information is individually identifiable under the Common Rule. The HIPAA rule considers PHI as any information that may identify an individual; was created or received by a member of a HIPAA covered entity; and relates to the individual's past, present, or future physical/mental health or condition, health care, or payment for health care. HIPAA recognizes two methods for de-identification of data.

Popular Posts:
- 1. indian health center patient portal
- 2. wholeness center fort collins patient portal
- 3. patient portal ascension waco
- 4. belleville memorial ospital patient portal
- 5. medfusion.net patient login
- 6. children's clinic east patient portal help
- 7. nea baptist patient portal + jonesboro arkansas
- 8. www.wrhs.com patient portal button
- 9. doctor report on patient that is diagnosis with hep c
- 10. superior ne doctors office patient portal