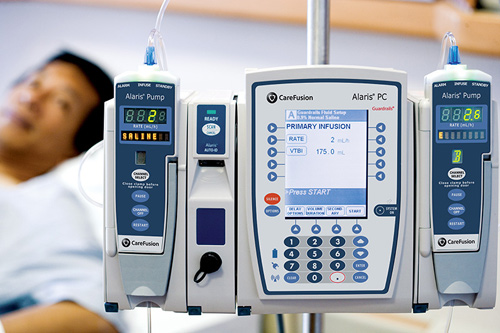
BD Alaris™ Pump Module
11 hours ago A large volume infusion pump that continuously or intermittently delivers fluids, medications, blood and blood products to adult, pediatric or neonatal patients. With the BD Alaris™ pump module, clinicians can attach up to four infusion modules, allowing four independent infusions on a single BD Alaris™ PC unit. Video Player is loading. >> Go To The Portal
What is the user manual for the Alaris pump and syringe?
General Information Pump and Syringe Modules Section 2-162 Alaris System User Manual – with v9.19 Model 8015 In this instrument, as with all infusion systems, the action of the pumping mechanism and variations in individual administration sets cause short-term fluctuations in rate accuracy.
Is there a recall on Alaris pumps?
Patients who receive fluids or medications delivered by the affected Alaris Pump systems On March 3, 2021, CareFusion 303, Inc. sent a New Urgent Medical Device Recall letter to all affected customers and provided the following instructions:
How do I enter a delay time on my Alaris pump?
RATE (mL/h) Pump and Syringe Modules Section General Programming Alaris System User Manual – with v9.19 Model 8015 2-107 2. Press Delay forsoft key. 3. To enter number of minutes (up to 120) infusion is to be delayed for, use numeric data entry keys. 4. Press CONFIRMsoft key. • Delay period counts down on Main Display.
What is the rate accuracy of the Alaris pump module?
Specifications (Continued) Pump Module (Continued) Rate Accuracy:Rate accuracy of Alaris System is ±5% at rates between 1 and 999 mL/h and ±5.5% at rates <1 mL/h, 95% of the time with 95% confidence, under conditions listed below.
How is Alaris different from other smart pumps?
Some advantages of the Alaris Pump System included better safety features, modularity, intuitive user interface, versatility, and ease of use.
What are the different types of Alaris pumps?
Each Alaris pump system is modular, and you can configure it to serve specific purposes. Every system is centralized to the PCU (point-of-care unit...
What is a module?
The Alaris System features a number of modules that allow the machine to be configured for specific purposes such as delivering pain medication, as...
What is a PCU?
A point-of-care unit (PCU) is the central component of the Alaris Pump System.
What is Guardrails?
Guardrails is a state-of-the-art comprehensive safety software built into every Alaris PCU. It allows healthcare professionals to customize various...
How many modules can be connected to the PCU?
There can be one to four modules attached to the PCU at any time.
Where are the Alaris pump modules most commonly used?
The Alaris pump system is used in many different healthcare facilities for large volume infusions in a variety of administration methods such as in...
What is BD Alaris?
The BD Alaris Pump Module System is an infusion pump and vital signs monitoring system that consists of a PC Unit, the Guardrails Suite MX, and up to four removable infusion or monitoring modules (channels). The System is used to deliver fluids including medications, blood, or blood products, into a patient's body in a controlled manner. It is used in hospitals and other health care facilities.
Why is Carefusion 303 recalling?
This could lead to keys that become unresponsive or stuck (See Figure 1). This may lead to an infusion delay or interruption or prevent clinicians from changing fluid or medication infusions on the affected devices.
How to contact BD for a recall?
To schedule remediation of the affected devices at your facility, contact BD at 1-888-562-6018. A BD service technician will visit your facility and perform keypad replacements of affected devices at no charge. Complete and return the Customer Response Form to acknowledge receipt of the notification and the recall instructions.
What is a Class I recall?
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
What is a pump module?
Pump modules are typically used throughout healthcare facilities for large volume infusions and indicated for use on adults, pediatrics and neonates through clinically acceptable routes of administration such as: intravenous (IV), intra-arterial (IA), subcutaneous, epidural, enteral or irrigation of fluid spaces.
Does Alaris pump cause hemolysis?
Yes. The Alaris Pump module causes no clinically significant hemolysis while infusing red cells or platelets. The following are some of the blood administration sets that can be used to administer blood products:
Can you use Alaris pump administration set?
Yes, the Alaris Pump module administration sets were developed for specific use with the Alaris Pump module. By manufacturing both the infusion devices and applicable administration sets we can ensure and confirm the stated accuracy flow rates. BD offers a wide variety of dedicated IV administration sets for the Alaris Pump module. Use of any other sets may cause improper instrument operation, resulting in an inaccurate fluid delivery or other potential hazard.
Is Alaris pump recommended?
No. The Alaris System is not recommended in this use. Even though the Alaris System’s accuracy is not affected by pressures of +300 mmHg for the Alaris Pump module, we cannot recommend specific therapies. ECMO is a therapy used in hospitals and our testing and documentation is not therapy-specific.
How does Alaris pump work?
Alaris smart pumps help increase safety for patients, clinicians and hospitals. They reduce infusing errors, reduce stress and help make sure the patient is always receiving the best care with the latest technology free from infusion errors.
What is Alaris 8100?
The Alaris 8100 can be updated wirelessly to have the latest drug and IV parameters and other important safety enhancements. The 8100 includes dose rate calculations and other advanced features.
Do Alaris modules need batteries?
It is just another terminology used to describe the system. Modules have no cords or batteries so it isn’t necessary to charge them or have to have them plugged in. However a module must be connected to an Alaris PCU to function.

Recalled Product
- BD Alaris Infusion Pump Module
- Affected Model Number: 8100
- Affected Parts: 49000239; 49000346; 49000438; 49000439
- Manufacturing Dates: 01/15/2019 to 12/05/2019.
Device Use
- The BD Alaris Pump Module System is an infusion pump and vital signs monitoring system that consists of a PC Unit, the Guardrails Suite MX, and up to four removable infusion or monitoring modules (channels). The System is used to deliver fluids including medications, blood, or blood products, into a patient's body in a controlled manner. It is used in hospitals and other health car…
Reason For Recall
- CareFusion 303 Inc is recalling the Alaris Pump Module because of the risk of the keypad lifting up due to fluid entry. This could lead to keys that become unresponsive or stuck (See Figure 1). This may lead to an infusion delay or interruption or prevent clinicians from changing fluid or medication infusions on the affected devices. High-risk pati...
Who May Be Affected
- Health care providers using the affected Alaris Pump System
- Patients who receive fluids or medications delivered by the affected Alaris Pump systems
What to Do
- On March 3, 2021, CareFusion 303, Inc. sent a New Urgent Medical Device Recall letter to all affected customers and provided the following instructions:
Contact Information
- Consumers with questions about this recall may contact BD by phone at (888) 562-6018, Monday through Friday between 7:00am and 4:00pm (Pacific Time) or by emailing SupportCenter@bd.com.
Additional Resources
- Medical Device Recall Database Entry
- BD Alaris System Recall Notification ResourcesExternal Link Disclaimer
How Do I Report A Problem?
- Health care professionals and consumers may report adverse reactions or quality problemsthey experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program using an online form, regular mail, or FAX.
Popular Posts:
- 1. georgia highlands medical patient portal
- 2. lewis gale patient portal login
- 3. sample patient profile report
- 4. my health upmc patient portal
- 5. bedside report and two-nurse safety check: a patient safety initiative
- 6. bergen medical associates patient portal
- 7. boone health connection patient portal
- 8. polk pediatrics patient portal
- 9. patient report persistent cough
- 10. central maine medical patient portal